Organic Electrochemical Transistors for Biosensing: Principles, Advances, and Clinical Applications
This article provides a comprehensive overview of Organic Electrochemical Transistors (OECTs) as a transformative technology in biosensing.
Organic Electrochemical Transistors for Biosensing: Principles, Advances, and Clinical Applications
Abstract
This article provides a comprehensive overview of Organic Electrochemical Transistors (OECTs) as a transformative technology in biosensing. Tailored for researchers, scientists, and drug development professionals, it explores the fundamental operating principles of OECTs, including the Bernards model and ionic/electronic charge coupling. The review delves into cutting-edge fabrication methodologies, such as flexible printed circuit board (fPCB) and inkjet printing for scalable, low-cost production. It critically examines device functionalization strategies—including gate, channel, and electrolyte modification—for detecting targets from small molecules to proteins and DNA. The content further addresses key challenges in sensor stability, reusability, and integration into wearable and point-of-care platforms, offering troubleshooting and optimization insights. By synthesizing recent advances and performance validation, this article serves as a foundational resource for developing next-generation bioelectronic devices for clinical diagnostics and personalized medicine.
The Foundation of OECTs: Unraveling Principles, Materials, and Biosensing Mechanisms
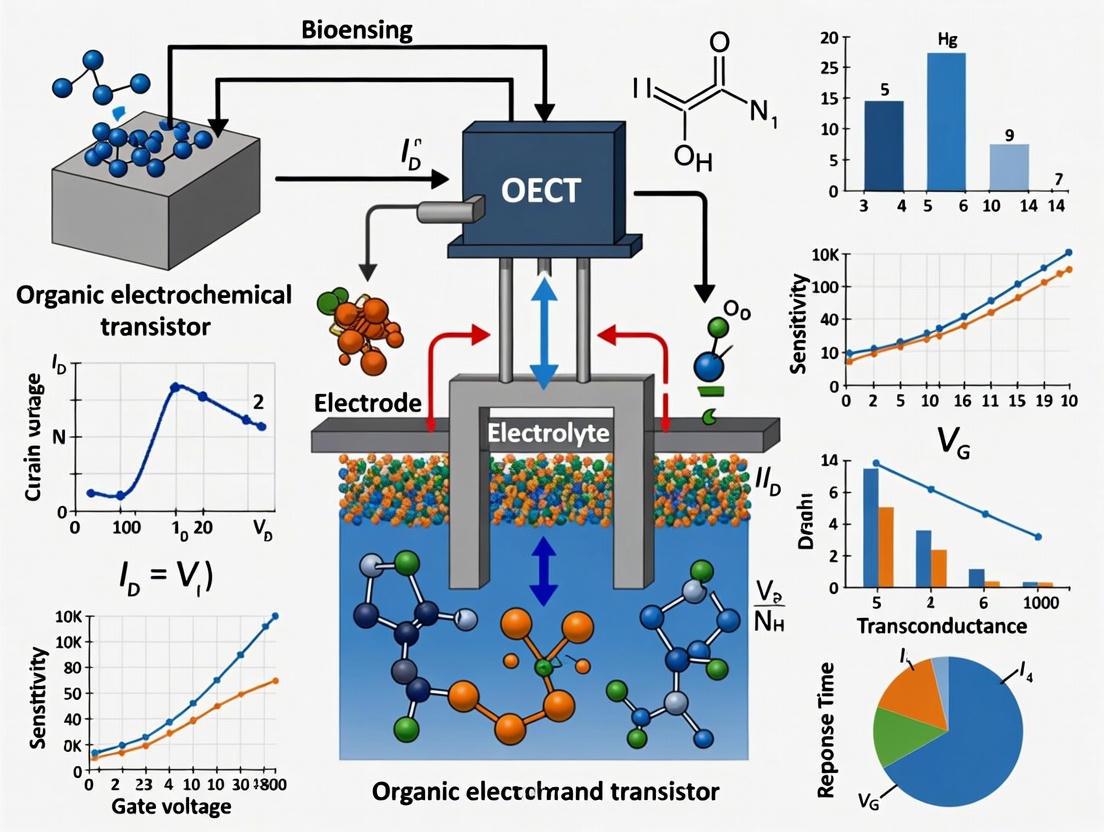
The organic electrochemical transistor (OECT) has emerged as a premier transducer in bioelectronics, renowned for its ability to effectively interface biological environments with electronic readout systems. Its operation hinges on the mixed ionic and electronic conduction within an organic semiconductor channel, allowing it to convert ionic fluxes from biological events into amplified electronic signals [1] [2]. This unique capability, combined with low operational voltages (typically < 1 V), inherent biocompatibility, and mechanical flexibility, makes the OECT an exceptionally versatile platform for a wide range of applications, from biosensing and neuromorphic computing to electrophysiological recording [3] [4] [5].
At the heart of the OECT's functionality is its fundamental structure, comprising three electrodes—gate, source, and drain—and an organic semiconductor channel, all interfaced through an electrolyte [2]. This configuration facilitates a bulk interaction between ions from the electrolyte and electronic charges in the channel, leading to high transconductance (gm), a key metric for signal amplification [1]. This article provides detailed application notes and experimental protocols centered on the basic OECT configuration, providing researchers and drug development professionals with the foundational knowledge and methodologies necessary to leverage this technology in advanced biosensing research.
OECT Device Architecture and Operational Principle
Core Components and Configuration
A typical OECT is constructed from several key components, each playing a critical role in its operation. The spatial arrangement of these components can be adapted into various architectures (e.g., planar, stacked) to suit specific application needs [1].
- Channel: The channel, typically a thin film of an organic mixed ionic-electronic conductor (OMIEC), bridges the source and drain electrodes. Its conductivity is modulated by the injection of ions from the electrolyte. The most common channel material is the conducting polymer poly(3,4-ethylenedioxythiophene):poly(styrene-sulfonate) (PEDOT:PSS), which operates in depletion mode [2]. Other OMIECs, including both p-type and n-type materials, are also employed [5].
- Source and Drain Electrodes: These are electronically conducting contacts (often made of gold or other inert metals) that allow a drain current (I
DS) to flow through the channel. The source is typically held at ground potential [2]. - Gate Electrode: The gate electrode is in ionic contact with the channel via the electrolyte. It can be a polarizable electrode (e.g., Pt, Au) or a non-polarizable electrode (e.g., Ag/AgCl). Applying a voltage (V
GS) to the gate electrode drives ions into or out of the channel [5] [1]. - Electrolyte: The electrolyte, which can be a liquid, gel, or solid, contains mobile ions and serves as the dielectric medium connecting the gate and the channel [1]. Its composition and concentration can significantly influence device performance.
The following diagram illustrates the operational principle of a standard depletion-mode OECT, such as one based on PEDOT:PSS.
Operational Mechanism and Sensing Principle
The operation of an OECT can be understood through the interplay of two coupled circuits: an electronic circuit (source-channel-drain) and an ionic circuit (gate-electrolyte-channel) [3]. The channel acts as a resistor whose conductivity is governed by its doping level, which is in turn controlled by the migration of ions from the electrolyte.
In a classic depletion-mode OECT using PEDOT:PSS:
- At zero gate voltage (V
GS= 0 V), the channel is highly conductive (doped, or oxidized, in the case of PEDOT:PSS), and a significant drain current (IDS) flows. This is the ON state [2]. - Upon application of a positive gate voltage (V
GS> 0 V), cations from the electrolyte are driven into the bulk of the OMIEC channel. This influx of ions dedopes (reduces) the p-type semiconductor, decreasing its hole density and conductivity, thereby switching the transistor to the OFF state [4] [2].
The efficiency of this gating process is quantified by the transconductance, gm = ∂IDS/∂VGS, which represents the amplification capability of the device. The Bernards and Malliaras model provides a foundational framework for understanding OECT operation, linking gm to material properties and device geometry [5] [3].
For biosensing, the presence or reaction of a target analyte can be transduced into an electrical signal through several mechanisms, primarily by affecting the effective gate voltage or the channel's doping state [5]. The most common strategy is gate functionalization, where a biorecognition element (e.g., an enzyme, antibody, or aptamer) is immobilized on the gate electrode. When the target analyte interacts with this functionalized gate, it induces a change in the gate potential, which modulates IDS, allowing for highly sensitive detection [5] [6].
Performance Characteristics and Key Metrics
The performance of an OECT is evaluated through its electrical characteristics and key figures of merit, which are crucial for benchmarking and optimizing devices for specific applications.
Table 1: Key Performance Metrics for OECT Characterization
| Metric | Symbol | Description | Measurement Method | Typical Values/Influencing Factors |
|---|---|---|---|---|
| Transfer Characteristics | IDS vs. VGS |
Shows the dependence of drain current on gate voltage at a constant drain voltage (VDS). |
Sweep VGS while measuring IDS at fixed VDS. |
Used to determine operating regime and ION/IOFF ratio [4]. |
| Output Characteristics | IDS vs. VDS |
Shows the dependence of drain current on drain voltage at different fixed gate voltages. | Sweep VDS while measuring IDS at fixed VGS steps. |
Reveals the transistor's saturation behavior [4]. |
| Transconductance | gm |
Figure of merit for signal amplification efficiency; the rate of change of IDS with respect to VGS. |
gm = ∂IDS/∂VGS, derived from transfer curve. |
High gm (>10 mS reported) is desirable for sensitive detection [5] [1]. Governed by µC*, geometry (W, L, d) [5]. |
| On/Off Ratio | ION/IOFF |
The ratio between the maximum and minimum channel current. | Extracted from the transfer curve. | Values ~1000 are achievable, important for switching applications [4]. |
| Volumetric Capacitance | C* | The capacitance per unit volume of the channel material, indicating its ion uptake capacity. | Calculated from OECT response or measured via electrochemical impedance spectroscopy [1]. | A high C* is critical for achieving high gm [1]. |
| Response Time | Ï„ | The speed at which the OECT switches between ON and OFF states. | Measured from the transient current response to a gate voltage pulse. | Ranges from microseconds (liquid electrolytes) to slower times (gels); limited by ion transport [3] [2]. |
| Cycle Stability | - | The ability of the device to maintain performance over repeated gating cycles. | Monitor transfer curves or transient response over many cycles (e.g., 60+ cycles). | Indicates operational robustness and is influenced by material stability and ion gel electrolytes [4]. |
Table 2: Impact of OECT Design Parameters on Device Performance
| Parameter | Impact on Performance | Considerations for Biosensing |
|---|---|---|
| Channel Dimensions (W, L, d) | gm ∠(W⋅d)/L [5]. A thicker, wider, and shorter channel maximizes gm. |
Miniaturization (small W, L) is key for high-density arrays and spatial resolution in sensing [4]. |
| Gate Electrode Type & Area | Non-polarizable gates (Ag/AgCl) allow for smaller gate areas. Polarizable gates (Au, Pt) require a large area (Cgate >> Cchannel) [5] [6]. |
The gate is often the functionalization site. A large, porous gate can enhance sensitivity [5]. |
| Electrolyte Composition | Ionic concentration and type affect mobility, Debye length, and device kinetics. | Biological fluids (saliva, sweat, blood) serve as electrolytes, making OECTs suitable for direct monitoring [1] [7]. |
| Channel Material | OMIEC properties (µ, C*) directly define gm and speed. |
PEDOT:PSS is common; new OMIECs are developed for stability, n-type operation, and specific sensing functions [5] [1]. |
Experimental Protocols for OECT Fabrication and Characterization
This section provides a detailed methodology for fabricating a flexible OECT array using a hybrid fPCB and inkjet printing approach, and for characterizing its critical performance metrics.
Protocol: Fabrication of Flexible OECT Arrays via fPCB and Inkjet Printing
This protocol outlines a rapid, low-cost method for fabricating flexible, all-solid-state OECTs, suitable for wearable biosensing applications [4].
Research Reagent Solutions and Materials
Table 3: Essential Materials for fPCB-based OECT Fabrication
| Item | Specification / Function |
|---|---|
| Flexible PCB Substrate | Polyimide (PI) substrate with photolithographically patterned Cu electrodes (thickness: ~35 µm). Serves as the mechanical support and source/drain/gate interconnects [4]. |
| Gold Plating Solution | Used to electroplate a thin layer (~20 nm) on Cu electrodes to protect against redox reactions and improve stability [4]. |
| PEDOT:PSS Ink | Commercially available or customized conducting polymer dispersion. Forms the OMIEC channel. Crosslinkers (e.g., GOPS) may be added to improve adhesion to the substrate [4]. |
| Gel Electrolyte | Non-aqueous poly(ethylene glycol) acrylate (PEA) based gel. Serves as the solid-state ion reservoir, prevents leakage and enhances mechanical stability [4]. |
| Inkjet Printer | Customizable piezoelectric printer for patterning the PEDOT:PSS channel and gel electrolyte with precise alignment (feature size down to 100 µm) [4]. |
| Encapsulation Layer | A second layer of polyimide (PI) to insulate the interconnects and define the active areas [4]. |
Step-by-Step Procedure
fPCB Electrode Fabrication:
- Pattern source, drain, gate electrodes, and interconnects on a polyimide substrate using standard photolithographic fPCB manufacturing processes.
- Electroplate a 20 nm gold layer onto the copper traces to enhance electrochemical stability.
- Apply a second polyimide layer to encapsulate the interconnects, leaving the electrode contact pads and active areas exposed [4].
Channel Patterning via Inkjet Printing:
- Prepare the PEDOT:PSS ink, optionally adding a crosslinker like (3-Glycidyloxypropyl)trimethoxysilane (GOPS) to a concentration of 1% v/v to improve film formation and adhesion.
- Using the inkjet printer, precisely deposit the PEDOT:PSS ink to form the transistor channel between the source and drain electrodes. A typical channel dimension is 100 µm (length) x 100 µm (width).
- Cure the printed PEDOT:PSS film according to the manufacturer's specifications (e.g., on a hotplate at 120°C for 60 minutes) to remove residual solvents and complete the crosslinking process [4].
Solid-State Electrolyte Patterning:
- Prepare the gel electrolyte precursor (e.g., PEA with ionic liquid or salt).
- Inkjet print the gel electrolyte onto the device to cover the overlap between the gate electrode and the OMIEC channel.
- Expose the device to UV light for polymerization to form the final solid-state gel electrolyte [4].
Quality Control:
- Inspect the fabricated devices under an optical microscope to check for printing defects, misalignment, or short circuits.
- Electrically test each unit in the array for basic functionality before proceeding to full characterization.
The following workflow diagram summarizes the fabrication process.
Protocol: Electrical Characterization of OECT Performance
This protocol describes the standard procedures for characterizing the steady-state and dynamic performance of an OECT.
Equipment and Software
- Source Measure Unit (SMU) or Potentiostat with multiple channels.
- Probe station for connecting to the OECT terminals.
- Faraday cage (recommended for low-noise measurements).
- Computer with data acquisition and analysis software (e.g., LabVIEW, Python, or MATLAB).
Step-by-Step Characterization Procedure
Setup:
- Place the OECT in a Faraday cage to minimize electrical noise.
- Connect the source, drain, and gate electrodes to the SMU.
- If using a liquid electrolyte, ensure the gate and channel are properly immersed. For gel electrolytes, ensure good contact.
Output Characteristics (I
DSvs. VDS):- Set the gate voltage (V
GS) to a constant value (e.g., 0 V). - Sweep the drain voltage (V
DS) from 0 V to a predetermined maximum (e.g., -0.6 V for a PEDOT:PSS OECT) while measuring the resulting drain current (IDS). - Repeat this sweep for several different, fixed V
GSvalues (e.g., 0 V, 0.2 V, 0.4 V, 0.6 V) [4].
- Set the gate voltage (V
Transfer Characteristics (I
DSvs. VGS) and Transconductance (gm):- Set the drain voltage (V
DS) to a constant value within the device's operational range (e.g., -0.3 V). - Sweep the gate voltage (V
GS) through the relevant voltage window (e.g., from -0.2 V to +0.8 V) while measuring IDS. - The transconductance (g
m) is calculated as the numerical derivative of the IDS-VGScurve. Plot gmas a function of VGSto identify the gate voltage for maximum amplification [4] [5].
- Set the drain voltage (V
Transient Response and Switching Speed:
- Apply a constant drain voltage (V
DS). - Apply a square-wave pulse train to the gate electrode. The pulse should alternate between voltages corresponding to the ON and OFF states (e.g., 0 V and 0.6 V), with a defined pulse width and frequency.
- Measure the resulting I
DSover time. The response time (Ï„) is typically defined as the time taken for the current to change from 10% to 90% of its maximum swing (or vice versa) upon the application of a gate pulse [4].
- Apply a constant drain voltage (V
Cyclic Stability Test:
- Continuously cycle the gate voltage (e.g., for 60 cycles or more) as in step 3 or apply repeated gate pulses as in step 4.
- Monitor the change in I
DSor the transfer curve over these cycles to assess the device's operational stability and reversibility [4].
Flexibility and Bending Tests (for flexible devices):
- Mount the OECT on testbeds with known, varying bending radii.
- Repeat the transfer characteristic measurements (Step 3) at each bending state.
- Compare key parameters (e.g., I
DS, gm) in the flat and bent states to evaluate mechanical robustness [4].
Troubleshooting and Common Experimental Challenges
Even with a standardized protocol, researchers may encounter specific issues during OECT fabrication and characterization. The table below outlines common problems, their potential causes, and recommended solutions.
Table 4: OECT Troubleshooting Guide
| Problem | Potential Causes | Suggested Solutions |
|---|---|---|
| High Device-to-Device Variation | Inconsistent channel printing; non-uniform electrolyte deposition; substrate surface contamination. | Optimize inkjet printing parameters (waveform, drop spacing); implement surface plasma treatment prior to printing; statistically characterize large arrays [4]. |
| Poor Operational Stability / Hysteresis | Electrochemical degradation of materials; delamination of functional layers; unstable gate electrode. | Use gel electrolytes to protect metal electrodes (e.g., Cu) [4]; incorporate crosslinkers in polymer inks [4]; use stable gate materials like Ag/AgCl or large surface area PEDOT:PSS gates [5]. |
| Slow Transient Response | Slow ion transport in the channel material or gel electrolyte; large channel dimensions. | Use OMIECs with high ionic mobility; reduce channel thickness and length; consider liquid electrolytes for faster operation [3] [1]. |
Low Transconductance (gm) |
Poor OMIEC properties (low µ or C*); suboptimal device geometry; inappropriate gate electrode. | Increase channel volumetric capacitance (C*); optimize geometry (increase W⋅d/L) [5]; ensure gate capacitance is much larger than channel capacitance [6]. |
| Noisy Signal | Unstable electrical connections; fluctuating electrochemical reactions; environmental interference. | Ensure good contact with probes; use a Faraday cage; employ stable, non-polarizable gate electrodes where possible [1]. |
The Bernards Model (also referred to as the Bernards-Malliaras model) provides a foundational theoretical framework for understanding the operating principles of Organic Electrochemical Transistors (OECTs) [8]. This model is particularly crucial for interpreting how OECTs transduce ionic signals from an electrolyte into electronic currents in a semiconductor channel, a core mechanism that makes OECTs exceptionally suitable for biosensing applications [1]. The model simplifies the complex electrochemical processes into more familiar electrical components, treating the OECT as two coupled circuits: an ionic circuit and an electronic circuit [8].
For researchers in biosensing and drug development, this conceptual separation is powerful. It enables the quantitative analysis of device parameters, allowing for the design of highly sensitive biosensors that can detect biomarkers, neurotransmitters, and other biological analytes [1] [9]. The model explains the unique amplification capability of OECTs, where a small ionic flux (e.g., from a biochemical binding event) can modulate a large electronic current, thereby providing highly sensitive detection of weak biological signals [1].
Theoretical Foundations of the Model
Core Principles and Circuit Decoupling
The Bernards Model rests on several key physical assumptions that allow for the decoupling of ionic and electronic transport [8]. First, it posits that ions injected from the electrolyte into the organic mixed ionic-electronic conductor (OMIEC) channel do not undergo Faradaic reactions with the polymer material itself. Instead, they act as immobile counter-ions that electrostatically compensate for electronic charges. This process is fundamentally capacitive in nature.
The model therefore conceptualizes an OECT as consisting of two distinct sub-circuits:
- The Electronic Circuit: The flow of electronic charge (holes in p-type devices) through the semiconducting polymer channel from the source to the drain electrodes. This circuit is treated as a simple resistor, where the conductivity is modulated by the presence of ions.
- The Ionic Circuit: The movement of ions within the electrolyte and their injection into the channel material. The channel itself, where ions compensate for electronic charges, is modeled as a bulk capacitance [8].
At steady state, when the gate voltage is applied and ions have finished moving into the channel, this capacitive element becomes fully charged, and the gate current drops to zero. This decoupled approach has been highly successful in fitting the steady-state output characteristics of OECTs.
Governing Equations and Key Parameters
For a p-type OECT operating in depletion mode, the Bernards Model describes the channel current (ICH) with a piecewise function, similar to a MOSFET model [8]:
For VD > VG - VT: ICH = μC* (W/d/L) [ (VT - VG) + (1/2)VD ] VD
For VD < VG - VT: ICH = - μC* (W/d/L) [ (VG - VT)2 / 2 ]
Where:
- μ is the charge carrier mobility (cm² Vâ»Â¹ sâ»Â¹)
- C* is the volumetric capacitance (F cmâ»Â³) of the channel material
- W, d, L are the width, thickness, and length of the channel, respectively
- VG and VD are the gate and drain voltages, respectively
- VT is the threshold voltage
A central figure of merit derived from this model is the transconductance, gm, which quantifies the amplification capability of the OECT. It is defined as the derivative of the channel current with respect to the gate voltage (gm = ∂ICH/∂VG). The product μC* is a critical material property that determines the ultimate performance of the OECT [8].
Table 1: Key Parameters in the Bernards Model and Their Physical Significance
| Parameter | Symbol | Unit | Physical Significance | Impact on OECT Performance |
|---|---|---|---|---|
| Volumetric Capacitance | C * | F cmâ»Â³ | Measures the capacity of the channel to store ions per unit volume. | Higher value enables greater current modulation and higher transconductance. |
| Charge Carrier Mobility | μ | cm² Vâ»Â¹ sâ»Â¹ | Describes how quickly electronic charges can move through the material. | Higher value leads to faster electronic response and higher current. |
| Geometry Factor | Wd/L* | Unitless | Aspect ratio of the OECT channel. | A larger Wd/L* increases the magnitude of the channel current. |
| Transconductance | gm | S | Ratio of output current change to input voltage change. | Key metric for signal amplification; higher is better for sensitive biosensing. |
Experimental Validation and Protocol
Validating the Bernards Model requires characterizing the steady-state electrical performance of an OECT. The following protocol outlines the key measurements and the procedure for extracting critical parameters.
Research Reagent Solutions and Materials
Table 2: Essential Materials for OECT Fabrication and Characterization
| Item | Function/Description | Example Specifications |
|---|---|---|
| Conducting Polymer | Forms the OMIEC channel; transports electronic charges and hosts ions. | PEDOT:PSS dispersion; p(g2T-T) or BBL for n-type operation [4] [10]. |
| Electrolyte | Medium for ionic transport; bridges the gate and the channel. | Aqueous saline (e.g., 0.1 M NaCl), gel electrolyte (e.g., PEA-based), or ionic liquid [4] [1]. |
| Gate Electrode | Provides the input voltage to drive ions into the channel. | Au, Pt, or Ag/AgCl for stable electrochemical window [1]. |
| Substrate | Mechanical support for the OECT. | Rigid (e.g., glass) or flexible (e.g., polyimide, fPCB) [4]. |
| Source/Drain Electrodes | Provide ohmic contact for electronic current injection/collection. | Au or Au-coated electrodes (e.g., on fPCB) to ensure stability [4]. |
| Crosslinker | Enhances adhesion of polymer and gel layers to substrates, improving mechanical stability. | Added to PEDOT:PSS or gel electrolytes for flexible devices [4]. |
Protocol: OECT Characterization and Parameter Extraction
Step 1: Device Fabrication
- Substrate Preparation: Begin with a cleaned and patterned substrate. Flexible printed circuit board (fPCB) technology offers a rapid, low-cost method for fabricating source, drain, and gate electrodes on a polyimide substrate [4].
- Channel Patterning: Deposit the OMIEC channel material (e.g., PEDOT:PSS) between the source and drain electrodes. Inkjet printing is a scalable method suitable for this purpose. For all-solid-state devices, add a crosslinker to the polymer solution to improve adhesion and prevent delamination [4].
- Electrolyte Integration: For gel-gated OECTs, deposit the gel electrolyte (e.g., PEA-based) over the channel and gate electrode using a customizable inkjet printer, ensuring proper alignment [4].
Step 2: Output and Transfer Curve Measurement
- Output Characteristics: Sweep the drain voltage (VD) from 0 V to a maximum value (e.g., -0.6 V for a p-type device) while stepping the gate voltage (VG) through a range of values (e.g., from 0.2 V to 0.8 V). Measure the resulting channel current (ICH) [4].
- Transfer Characteristics: At a fixed drain voltage (e.g., VD = -0.3 V), sweep the gate voltage and measure the channel current. This curve is used to extract the transconductance.
Step 3: Data Analysis and Parameter Extraction
- Extract Transconductance (gm): Calculate gm by differentiating the transfer curve (ICH vs. VG) at a specific VD.
- Calculate Mobility (μ) and Capacitance (C*): Fit the output characteristic data to the Bernards Model equations (Section 2.2). The mobility and volumetric capacitance can be extracted from this fitting procedure [4] [8]. For example, a reported fPCB-fabricated OECT exhibited a mobility of 1.1 cm² Vâ»Â¹ sâ»Â¹ calculated using this model [4].
Applications in Biosensing and Beyond
The Bernards Model provides the theoretical backbone for designing and optimizing OECTs for a wide range of applications, particularly in biosensing.
- High-Sensitivity Biomarker Detection: The model's focus on transconductance guides the design of OECTs for amplifying weak electrical signals from biological binding events. This has enabled the detection of neurotransmitters like catecholamine at nanomolar concentrations [1], and the sensing of glucose in sweat and other biofluids using enzymatic or molecularly imprinted polymer (MIP) functionalized gates [11] [9].
- Neuromorphic Computing: The ionic dynamics described by the Bernards Model are harnessed to create devices that mimic synaptic behavior. The transient response of the channel current to gate voltage pulses can emulate short-term and long-term plasticity, forming the basis for artificial synapses and organic electrochemical random-access memories (ECRAMs) [8]. Recent work on flexible, all-gel OECTs has demonstrated their use in neuromorphic simulation for tactile perception in robotic hands [10].
- Wearable and Implantable Sensors: The understanding of device physics allows for the creation of OECTs on flexible substrates like fPCB or stretchable all-gel networks [4] [10]. These devices maintain performance under mechanical deformation, enabling their use as conformal biosensors for continuous health monitoring, such as lactate sensing in sweat to indicate muscle fatigue [9].
Current Limitations and Future Perspectives
While the Bernards Model is an excellent tool for predicting steady-state OECT behavior, it has limitations, particularly concerning transient response. The model's assumption of a purely capacitive ionic-electronic coupling does not fully capture the complex, time-dependent ion dynamics that govern switching speed and non-volatile memory behavior [8].
Future research is focused on bridging this gap by developing more comprehensive models that account for:
- Ion Transport Kinetics: The diffusion and drift of ions within the complex nano-morphology of the OMIEC, which can be the speed-limiting factor [8].
- Electrochemical Doping and Swelling: The impact of water uptake and volumetric swelling of the channel material on ion transport and electronic mobility [1].
- Structural Evolution: Real-time changes in the polymer's microstructure (e.g., π-π stacking distance) during operation that can alter transport properties [8].
Advances in operando characterization techniques and multi-physics modeling will be crucial to develop a more unified understanding, ultimately accelerating the design of next-generation OECTs for high-speed biosensing and neuromorphic computing applications.
Organic Electrochemical Transistors (OECTs) have emerged as a preeminent technology in biosensing due to their high signal-to-noise ratio, low operating voltage, and exceptional biocompatibility [12] [13]. The performance of OECT-based biosensors is critically dependent on their ability to efficiently convert a biological event into an amplified, measurable electrical signal. The central metric quantifying this amplification capability is transconductance (gm).
Transconductance, defined as gm = ∂ID/∂VG, represents the efficiency with which a small change in gate voltage (VG) modulates the drain current (ID) [13]. A higher transconductance signifies a greater amplification factor, enabling the sensor to detect weaker biological signals with higher sensitivity, which is paramount for detecting low-abundance biomarkers, neurotransmitters, and ions in complex physiological environments [14].
In OECTs, this amplification stems from a unique mixed ionic-electronic conduction process. When a gate voltage is applied, ions from the electrolyte migrate into the bulk of the organic semiconductor channel, modulating its doping level and electronic conductivity [15] [16]. This volumetric capacitance, distinct from the interfacial capacitance in traditional field-effect transistors, is the origin of OECTs' high transconductance and superior performance in aqueous, biological milieus [12].
Quantitative Relationship Between gm and Biosensor Performance
The sensitivity of an OECT biosensor is directly governed by its transconductance. The fundamental equation defining the maximum transconductance is [13]:
gm = (Wd / L) * μ * C* * (VTh - VG)
This equation reveals that gm is not an intrinsic property of the channel material alone but a function of both device geometry (W, d, L) and material properties (μ, C). The product μC serves as a valuable material quality factor, allowing for a geometry-independent comparison of different organic mixed ionic-electronic conductors (OMIECs) [13].
Table 1: Key Parameters Influencing OECT Transconductance and Sensor Sensitivity.
| Parameter | Symbol | Description | Impact on gm and Sensitivity |
|---|---|---|---|
| Channel Width | W | Width of the conducting channel. | Increasing W raises gm, enhancing current drive and signal amplitude [13]. |
| Channel Length | L | Distance between source and drain electrodes. | Decreasing L increases the W/L ratio, significantly boosting gm [13]. |
| Channel Thickness | d | Thickness of the organic semiconductor film. | Increasing d raises gm but can slow the device's response time [13]. |
| Carrier Mobility | μ | How quickly charges move through the material. | Higher μ leads to higher gm, improving switching speed and amplification [13]. |
| Volumetric Capacitance | C* | Ability of the channel to store ions per unit volume. | A higher C* enables a greater doping change per gate voltage, maximizing gm [12] [16]. |
| Material Quality Factor | μC* | Product of mobility and volumetric capacitance. | A high μC* is essential for achieving high-gm devices without relying solely on geometry [13]. |
For fiber-based OECTs (F-OECTs), the geometric considerations differ. The channel width W is determined by the circumference of the fiber (Ï€d), which is inherently larger than the width of a planar channel with the same footprint. This allows F-OECTs to achieve very high W/L ratios, resulting in superior transconductance and current-driving capability compared to their planar counterparts [12].
The ultimate sensitivity of a biosensor, often defined by its Limit of Detection (LOD), is a function of the signal-to-noise ratio. A high gm provides greater signal amplification for a given biological binding event, thereby lowering the LOD. This has been demonstrated in sensors for dopamine, where OECTs with optimized transconductance have achieved detection limits in the nanomolar range [14].
Experimental Protocols for Transconductance Characterization
Accurate characterization of transconductance is a prerequisite for developing and validating high-performance OECT biosensors. The following protocol details the standard measurement procedure.
Equipment and Reagent Setup
- OECT Device: A fabricated OECT with defined channel geometry (W, L, d).
- Source Measure Units (SMUs): A semiconductor parameter analyzer or a combination of two precision source-measure units to independently control VG and VD while measuring ID.
- Electrolyte: A suitable aqueous electrolyte, such as phosphate-buffered saline (PBS) at physiological concentration (e.g., 0.1 M), to mimic biological conditions [16].
- Electrochemical Cell: A probe station or a custom fluidic cell that allows stable contact between the electrolyte and the OECT's gate and channel.
- Faraday Cage: (Recommended) To minimize external electromagnetic interference during sensitive measurements.
Step-by-Step Measurement Procedure
- Device Immersion & Stabilization: Immerse the OECT's gate and channel in the electrolyte solution. Allow the device to stabilize for a predetermined period (e.g., 15-30 minutes) until the open-channel current (IDS) reaches a steady state, indicating stable electrolyte/channel interaction.
- Output Curve Measurement:
- Set the gate voltage (VG) to a starting value (e.g., 0 V).
- Sweep the drain voltage (VD) from 0 V to a low voltage, typically -0.6 V or -0.8 V, to avoid electrochemical side reactions.
- Record the corresponding drain current (IDS) at each VD step.
- Repeat this sweep for a series of VG values (e.g., 0 V, 0.2 V, 0.4 V, 0.6 V).
- Output: A family of IDS vs. VD curves used to verify proper transistor operation and identify the linear/saturation regimes.
- Transfer Curve Measurement & gm Extraction:
- Set the drain voltage (VD) to a constant value within the saturation region (e.g., -0.6 V).
- Sweep the gate voltage (VG) across the desired operating range (e.g., from 0 V to 0.8 V).
- Record the corresponding drain current (IDS) at each VG step.
- Data Processing: The transconductance (gm) is calculated as the first derivative of the transfer curve: gm = ∂IDS/∂VG. This is typically done using numerical differentiation of the IDS vs. VG data.
- Output: A transfer curve (IDS vs. VG) and its corresponding gm vs. VG plot, which identifies the peak transconductance (gm,max) and the optimal gate voltage for sensor operation.
- Validation with Model: For depletion-mode PEDOT:PSS OECTs, validate the data by fitting the transfer curve in the saturation region to the equation: IDS = [-q μ p0 t W / (2 L Vp)] * (VG - Vp)^2, where Vp is the pinch-off voltage [12].
Figure 1: Experimental workflow for the characterization of OECT transconductance.
The Scientist's Toolkit: Essential Research Reagents and Materials
The development of high-transconductance OECTs relies on a specific set of materials and reagents, each serving a critical function in device fabrication and operation.
Table 2: Key Research Reagent Solutions for OECT Biosensor Development.
| Category | Material/Reagent | Function in OECTs | Key Considerations |
|---|---|---|---|
| Channel Material | PEDOT:PSS | The most common p-type OMIEC; serves as the electroactive channel where doping modulation occurs [13]. | High conductivity formulations; often requires secondary doping (e.g., with ethylene glycol) for enhanced performance [13]. |
| N-type Polymers | e.g., BBL, p(g2T-TT), p(g2T-TTT) | Enable n-type (electron-conducting) OECT operation and the development of complementary logic circuits [15]. | Developing stable, high-mobility n-type materials in aqueous environments is a key research area [15]. |
| Liquid Electrolyte | Phosphate Buffered Saline (PBS) | Standard aqueous electrolyte for in-vitro testing; provides mobile ions (Na+, K+, Cl-) for gating the channel [12]. | Ion concentration and type influence capacitance and device dynamics [12]. Prone to evaporation [16]. |
| Gel Electrolyte | Polyvinyl Alcohol (PVA) / Salt composites | Solid-state electrolyte; prevents leakage, enhances device stability and flexibility for wearable applications [16]. | Ionic conductivity must be maintained; mechanical properties should match biological tissues for implants [16]. |
| Hydrogels | PEG, PHEMA, Chitosan | Biocompatible, tissue-like solid electrolytes; ideal for implantable and wearable biosensors due to their soft, hydrating nature [16]. | Can be engineered with self-healing, adhesive, or stimuli-responsive properties [16]. |
| Gate Electrode | Ag/AgCl, Gold (Au), Platinum (Pt) | The gate electrode applies the potential to the electrolyte. Ag/AgCl is a non-polarizable reference, while Au/Pt are polarizable [12] [14]. | Material choice affects gate capacitance and stability. Surface modification is often used to enhance selectivity [14]. |
| Functionalization Agent | 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE) | A common linker molecule for immobilizing biorecognition elements (e.g., antibodies) on graphene or carbon-based gate electrodes [17]. | Enables covalent bonding of biomolecules, creating a specific sensing interface on the device [17]. |
| 3α-Dihydrocadambine | 3α-Dihydrocadambine, MF:C27H32N2O10, MW:544.5 g/mol | Chemical Reagent | Bench Chemicals |
| Delavinone | Delavinone, MF:C27H43NO2, MW:413.6 g/mol | Chemical Reagent | Bench Chemicals |
Advanced Considerations and Material Design for Enhanced gm
Beyond geometric scaling, the strategic design of materials and device architecture is crucial for pushing the limits of transconductance and sensitivity.
Material Engineering for High μC*
The pursuit of a high material quality factor (μC*) is central to modern OECT research [13]. Innovations in organic semiconductor synthesis focus on:
- Improving Ionic Permeability: Designing polymer backbones with enhanced volumetric capacitance (C*) by facilitating easier ion penetration and more efficient electrochemical doping/dedoping [15].
- Enhancing Charge Transport Mobility (μ): Developing new conjugated polymers with more ordered microstructures and reduced energetic disorder to boost charge carrier mobility [13].
Device Architecture and Ion Transport
The physical structure of the OECT profoundly influences its performance. In fiber-based OECTs (F-OECTs), the three-dimensional architecture (e.g., twisted, coaxial) creates new pathways for ion transport. A mechanism known as Lateral Insertion-assisted ion transport allows ions to diffuse both vertically and laterally along the fiber, which can significantly reduce ion diffusion time and improve the device's response speed, thereby enhancing the effective gm for dynamic signals [15].
Operational Mode: Depletion vs. Accumulation
Most common PEDOT:PSS OECTs operate in depletion mode, where the channel is conductive at VG=0 and is switched "off" by a positive gate voltage [12] [15]. Conversely, accumulation-mode OECTs, often based on n-type polymers, start in an "off" state and are turned "on" by the gate voltage. Accumulation-mode devices are particularly advantageous for low-power applications and can achieve high ON/OFF ratios, which is beneficial for digital sensing and neuromorphic computing [15].
Organic Mixed Ionic-Electronic Conductors (OMIECs) are carbon-based materials capable of efficiently transporting and coupling both ionic and electronic charges, making them fundamental to the operation of Organic Electrochemical Transistors (OECTs) [18]. In OECTs, which are three-terminal devices, an OMIEC film forms the channel between the source and drain electrodes. This channel is in direct contact with an electrolyte, which serves as the gate dielectric. The application of a gate voltage drives ions from the electrolyte into the bulk of the OMIEC channel, thereby electrochemically modulating its doping state and electronic conductivity [15] [16]. This mixed conduction mechanism enables OECTs to excel in biosensing applications, offering high transconductance, low operating voltages, biocompatibility, and the ability to amplify small biological signals [19] [5].
Key OMIEC Materials and Their Performance
The performance of an OECT is largely dictated by the properties of its OMIEC channel material. The key figure of merit (FoM) for an OECT is the product of charge carrier mobility (µ) and volumetric capacitance (C*), which determines the transconductance and signal amplification capability [16]. Researchers have developed and characterized a wide range of OMIECs, from benchmark materials like PEDOT:PSS to novel synthetic polymers and blends.
Table 1: Comparison of Key OMIEC Channel Materials for OECTs
| Material | Type/Operation Mode | Key Performance Metrics | Advantages | Disadvantages |
|---|---|---|---|---|
| PEDOT:PSS [18] [20] [21] | p-type, Depletion | µC* ≈ 1500 F cmâ»Â¹ Vâ»Â¹ sâ»Â¹ [16]; Transconductance up to 11±3 mS [18] | High conductivity, biocompatibility, commercial availability, facile processing | Limited volumetric capacitance, requires crosslinking for stability in water |
| PEDOT:ClOâ‚„ [21] | p-type, Depletion | High cycling stability (>1000 cycles); Performance stable under mechanical strain [21] | Small, mobile dopant enables stable performance; Suitable for flexible electronics | Requires electropolymerization |
| Melanin/PEDOT:PSS Blend [18] | p-type, Depletion | Transconductance: 11±3 mS (vs. 7±1 mS for pure PEDOT:PSS); 10x increase in C* [18] | Enhanced ionic-electronic coupling; Sustainable, bio-inspired component | Processability challenges at high melanin concentrations |
| PANI:DBSA [20] | p-type | N/A | Inexpensive raw materials; Good film smoothness and reproducibility | Lower conductivity and transconductance than PEDOT:PSS |
| PBTI2g-DTCN [22] | n-type, Accumulation | µC* = 287.8 F cmâ»Â¹ Vâ»Â¹ sâ»Â¹; Electron mobility: 0.84 cm² Vâ»Â¹ sâ»Â¹ [22] | High-performance n-type OMIEC; Excellent operational stability | Synthetic complexity |
Detailed Experimental Protocols
This section provides standardized protocols for fabricating and characterizing OECTs with different OMIEC channels, ensuring reproducibility and reliability in biosensing research.
Protocol 1: Fabrication of a Porous PEDOT:PSS OECT for 3D Cell Culture Monitoring
This protocol details the creation of a scaffold-like OECT with a porous PEDOT:PSS channel, ideal for hosting 3D cell cultures and monitoring cellular functions through ion fluxes and redox reactions [23].
Research Reagent Solutions:
- Channel Cocktail: 1.0 wt% PEDOT:PSS (e.g., Orgacon ICP 1050) and 3.0 wt% (3-glycidyloxypropyl)trimethoxysilane (GOPS) in water [23].
- Electrode Substrate: Glass substrate with patterned Au source/drain electrodes (100 nm thickness, using Cr as adhesive layer). Channel width (W) = 1000 µm, length (L) = 10 µm [23].
- Gate Electrode: Ag/AgCl reference electrode in saturated KCl connected via a salt bridge [23].
- Measurement Electrolyte: 1X Phosphate Buffered Saline (PBS), pH 7.4 [23].
Procedure:
- Substrate Preparation: Begin with a pre-patterned Au-on-glass substrate. Clean the substrate thoroughly to ensure good adhesion of the channel material.
- Channel Deposition: Deposit 5 µL of the prepared PEDOT:PSS/GOPS cocktail onto the channel area between the source and drain electrodes [23].
- Freezing: Immediately transfer the substrate to a -80 °C freezer and freeze for 1 hour. This step phase-separates the components to form the porous structure [23].
- Freeze-Drying: Place the frozen device in a freeze-dryer to remove the frozen solvent via sublimation, leaving a porous solid scaffold [23].
- Cross-Linking: Anneal the device at 120 °C for 1 hour to thermally cross-link the GOPS, stabilizing the porous structure and making it insoluble in aqueous solutions [23].
- Assembly: Attach a glass ring to the substrate to create a reservoir for the measurement solution or cell culture medium [23].
- Characterization: Use scanning electron microscopy (SEM) to confirm pore formation. The median pore diameter should be approximately 50 µm, suitable for cell infiltration [23].
Porous OECT Fabrication Workflow
Protocol 2: Fabrication of a High-Performance Planar PEDOT:PSS OECT
This protocol is optimized for producing high-quality, dense PEDOT:PSS films with excellent electrical characteristics for standard biosensing applications [20].
Research Reagent Solutions:
- PEDOT:PSS Solution: 5 mL commercial PEDOT:PSS dispersion (0.5-1 wt% in water).
- Additive Solutions: Ethylene glycol (150 µL, 3%), Dodecylbenzenesulfonic acid (DBSA, 12 µL, ~0.25%), and GOPS (50 µL, 1%) [20].
- Electrode Substrate: Glass slide with pre-patterned Au source/drain electrodes (L=130 µm, W=2 mm) [18].
Procedure:
- Solution Preparation: To 5 mL of PEDOT:PSS dispersion, add ethylene glycol and DBSA. Stir and sonicate the mixture for 10 minutes. Then, add 50 µL of GOPS and stir for 1 minute with sonication [20].
- Substrate Cleaning: Clean the electrode substrate with DI water. Render it hydrophilic by irradiating with ozone plasma for 20 minutes [20].
- Spin-Coating: Drop 75 µL of the prepared PEDOT:PSS solution onto the channel. Let it sit for 100 seconds without spinning, then spin-coat at 3000 rpm for 40 seconds [20].
- Annealing and Cross-Linking: Anneal the film on a hotplate at 135 °C for 1 hour [20].
- Post-Treatment (Optional): Immerse the coated electrode in DI water for 18 hours to remove impurities and low-molecular-weight components, resulting in a smoother film surface [20].
- Electrical Characterization: Measure the OECT's output and transfer characteristics in PBS using a semiconductor parameter analyzer. Calculate conductivity and transconductance from the linear region of the output curve at V_G = 0 V and the peak of the transfer curve, respectively [20].
Protocol 3: Creating a Melanin/PEDOT:PSS Blend for Enhanced Capacitance
This protocol describes blending synthetic melanin with PEDOT:PSS to create a more sustainable OMIEC with superior ionic-electronic coupling and volumetric capacitance [18].
Research Reagent Solutions:
- Synthetic Melanin (Mel): Synthesize water-soluble melanin by reacting 0.3 g of DL-DOPA in 60 mL Milli-Q water and 400 µL NH₄OH under 6 atm of O₂ for 6 hours. Purify via dialysis and dry at 90 °C [18].
- Mel/PEDOT:PSS Blends: Dilute the synthesized melanin powder directly into commercial PEDOT:PSS solution to create blends with 0, 10, 20, 30, and 50 wt% melanin content. Stir for 15 minutes and sonicate for 1 hour before film deposition [18].
Procedure:
- Material Synthesis: Follow the steps above to synthesize and purify water-soluble melanin [18].
- Blend Preparation: Create the desired Mel/PEDOT:PSS blends by weight. Note that blends with >50% melanin content show poor film processability [18].
- Device Fabrication: Fabricate OECTs using the spin-coating and annealing procedures outlined in Protocol 3.2.
- Performance Evaluation: Characterize the OECT performance. The optimal blend (e.g., 30% Mel) should show a significant increase in transconductance and volumetric capacitance compared to pure PEDOT:PSS devices [18].
Biosensing Applications and Mechanisms
OECTs functionalized with various OMIECs have been successfully deployed for a wide range of biosensing applications. The sensing mechanism typically relies on one of three strategies [5]:
- Gate Functionalization: The gate electrode is modified with receptors (e.g., enzymes, antibodies). The binding or reaction of the target analyte on the gate surface alters the effective gate potential, modulating the channel current [5].
- Channel-Electrolyte Interface Functionalization: The channel surface is functionalized so that the target analyte directly interacts with it, changing the channel's electronic structure or interface potential [5].
- Electrolyte Functionalization: The electrolyte itself is modified with sensing elements like enzymes or ion-selective membranes, which generate an ionic signal in response to the analyte that gates the transistor [5].
Table 2: OECT Biosensing Applications by Target Analyte
| Target Analyte | OMIEC Channel | Functionalization/Sensing Strategy | Reported Performance |
|---|---|---|---|
| Cations (Kâº, Naâº, etc.) [23] | Porous PEDOT:PSS | Direct gating by cation injection into the channel. | Current response to concentration changes from 1 mM to 1 M [23]. |
| Hydrogen Peroxide (Hâ‚‚Oâ‚‚) [23] | Porous PEDOT:PSS | Direct redox reaction with the PEDOT channel. | Current response to concentrations from 0.01 to 1.3 wt% [23]. |
| Glucose, Lactate [5] | PEDOT:PSS | Gate or electrolyte functionalized with corresponding oxidase enzyme (e.g., glucose oxidase). Enzyme reaction produces Hâ‚‚Oâ‚‚, which is detected. | High sensitivity and low detection limits achieved [5]. |
| Dopamine [5] | PEDOT:PSS | Gate functionalization; direct oxidation of dopamine at the gate alters effective V_G. | Capable of real-time monitoring in physiological fluids [5]. |
| DNA, Proteins [5] | PEDOT:PSS | Gate or channel functionalized with aptamers or antibodies. Binding events induce potential or capacitance changes. | High specificity for biomarker detection [5]. |
OECT Biosensing Mechanisms
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Materials and Reagents for OECT Research
| Reagent / Material | Function / Purpose | Example / Notes |
|---|---|---|
| PEDOT:PSS Dispersion | Benchmark p-type OMIEC; forms the conductive channel. | Sigma-Aldrich Orgacon ICP 1050; often requires additives [23] [20]. |
| (3-glycidyloxypropyl)trimethoxysilane (GOPS) | Cross-linker; renders PEDOT:PSS films insoluble in aqueous electrolytes. | Critical for stable operation in water [23] [20]. |
| Ethylene Glycol (EG) | Secondary dopant; enhances the electronic conductivity of PEDOT:PSS films. | Can increase conductivity by nearly two orders of magnitude [20]. |
| Dodecylbenzenesulfonic Acid (DBSA) | Dopant and surfactant; improves conductivity and film formation for PANI and PEDOT:PSS. | Used to process PANI into organic solvents [20]. |
| Polyaniline (PANI) | Alternative p-type OMIEC; lower cost but also lower performance than PEDOT:PSS. | Requires doping with acids like DBSA or CSA for solubility and conductivity [20]. |
| Ag/AgCl Gate Electrode | Non-polarizable gate; provides a stable reference potential, allowing for smaller gate designs. | Preferred over polarizable gates (Pt, Au) for integrated sensors [5]. |
| Gel Electrolytes | Solid-state electrolyte; prevents leakage, enables flexible/wearable devices. | Includes hydrogels (e.g., PVA) and ionic liquid gels (e.g., [Câ‚‚MIM][EtSOâ‚„]) [16]. |
| Propioxatin A | Propioxatin A, MF:C17H29N3O6, MW:371.4 g/mol | Chemical Reagent |
| Hrk BH3 | Hrk BH3, MF:C99H160N30O31, MW:2266.5 g/mol | Chemical Reagent |
PEDOT:PSS remains the cornerstone OMIEC for OECTs due to its excellent conductivity, ease of processing, and well-understood properties. However, as research advances, materials like melanin blends show promise for enhancing sustainability and performance, while novel n-type polymers like PBTI2g-DTCN are unlocking complementary circuits and low-power devices [18] [22]. The future of OMIEC development lies in creating tailored materials that offer not only high µC* products but also improved stability, specificity, and integration with solid-state electrolytes for robust, flexible, and implantable bioelectronic sensors. The combination of material innovation, precise fabrication protocols, and creative functionalization strategies will continue to expand the sensitive and powerful biosensing capabilities of OECTs.
Organic Electrochemical Transistors (OECTs) have emerged as a prominent platform in the field of bioelectronics, particularly for biosensing applications. Their significance stems from an efficient architecture that separates the gate electrode from the transistor device, enabling high-sensitivity detection of biological events [3]. OECTs operate at low voltages (typically below 1 V), exhibit high transconductance (gm), and possess inherent biocompatibility, making them exceptionally suitable for interfacing with biological systems [5] [3]. The core of an OECT's function lies in its use of an organic mixed ionic-electronic conductor (OMIEC) as the channel material, which facilitates the transduction of biological signals into amplified electrical readouts through electrochemical processes [5] [13]. This document delineates the fundamental mechanisms behind this transduction and provides detailed protocols for their experimental implementation, framed within the context of advanced biosensing research.
Fundamental Working Principles of OECTs
Device Structure and Configuration
A typical OECT is a three-terminal device consisting of source, drain, and gate electrodes [5] [13]. The source and drain electrodes are connected by a channel made from an OMIEC, such as the commonly used poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) [5] [13]. This channel is in direct contact with an electrolyte, which also contains the gate electrode. This configuration is a key differentiator from traditional organic field-effect transistors (OFETs), as the electrolyte replaces the insulating dielectric layer, allowing for direct ionic modulation of the channel's conductivity [3]. The gate electrode can be made from polarizable materials (e.g., Au, Pt) or non-polarizable materials (e.g., Ag/AgCl), with the latter often preferred for integrated devices as it avoids the need for an oversized gate [5].
Operational Mechanism and Transconductance
The operation of an OECT is governed by the electrochemical doping and de-doping of the channel material by ions from the electrolyte [3]. When a gate voltage (VG) is applied, it drives ions to move between the electrolyte and the OMIEC channel. For a p-type OECT like one based on PEDOT:PSS, applying a positive VG causes cations from the electrolyte to inject into the channel. This influx of cations electrochemically reduces the PEDOT chain (de-doping), decreasing the hole density and thus reducing the drain current (ID) [5] [16]. The relationship is described by the Bernards model, which treats the OECT as two coupled circuits: an ionic circuit (ions moving in the electrolyte) and an electronic circuit (holes or electrons moving in the channel) [5] [3].
The efficiency of an OECT in converting a small gate voltage change into a large current signal is quantified by its transconductance, gm = ∂ID/∂VG [5] [13]. This parameter is crucial for biosensing, as it directly correlates with the device's signal amplification capability and sensitivity. The maximum transconductance can be expressed as: [ gm = \frac{W d}{L} \mu C^* |V{G} - V_{T}| ] where W, d, and L are the channel width, thickness, and length, respectively; μ is the charge carrier mobility; C* is the volumetric capacitance of the channel material; and VT is the threshold voltage [5] [13]. Consequently, high-performance OECTs can be engineered by optimizing the device geometry and the OMIEC's electronic properties [16].
Core Biosensing Mechanisms and Functionalization Strategies
The exceptional sensitivity of OECTs to ionic and electrochemical changes in their environment is harnessed for biosensing through targeted functionalization. The primary sensing mechanisms can be classified into three categories, based on which component of the OECT is functionalized to interact with the target analyte.
Gate Functionalization
This is the most conventional and widely used strategy for developing OECT-based biosensors [5]. The gate electrode is modified with a biorecognition element (e.g., an enzyme, antibody, or aptamer) that is specific to the target analyte.
- Mechanism: When the target analyte interacts with the functionalized gate surface, it triggers a change in the gate's electrochemical properties. This can occur via:
- A redox reaction that generates or consumes electrons, altering the effective gate potential (VeffG) [5] [24]. For instance, the enzyme glucose oxidase catalyzes the oxidation of glucose, producing H2O2, which can be oxidized at the gate electrode, generating a current that modulates VG.
- A capacitive change caused by the binding of charged biomolecules (e.g., DNA, proteins) to the gate surface, which alters the double-layer capacitance and thus VeffG [5].
- Signal Transduction: The change in VeffG is then amplified by the OECT, resulting in a measurable shift in the transfer curve (ID vs. VG) or a change in the drain current at a fixed bias [5]. This mechanism is highly effective for detecting a wide range of targets, from small molecules like glucose and dopamine to macromolecules like DNA and proteins [5] [13].
Channel-Electrolyte Interface Functionalization
In this approach, the surface or bulk of the OMIEC channel is functionalized to be responsive to the target analyte.
- Mechanism: The analyte interacts directly with the channel material, leading to a change in the channel's electronic structure or a voltage drop at the electrolyte/channel interface [5]. This interaction can modulate the channel's conductivity directly, without necessarily involving the gate electrode.
- Signal Transduction: The binding or reaction event alters the doping level or charge carrier mobility within the channel, leading to a direct change in the drain current (ID) [5] [13]. This method is often used for sensing ions or pH, where the channel material itself can be designed to be selectively responsive.
Electrolyte Functionalization
The electrolyte itself can be turned into a sensing component by incorporating elements that react with the target.
- Mechanism: Enzymes, ion-selective membranes, or even suspended cells are integrated into the electrolyte [5]. The primary reaction with the analyte occurs within the electrolyte bulk. For example, an enzyme like lactate oxidase can be suspended in the electrolyte to catalyze the breakdown of lactate, producing a change in local ion concentration (e.g., H+).
- Signal Transduction: The products of the reaction (e.g., ions) modulate the ionic strength or composition of the electrolyte. This change affects the gating efficiency of the OECT, ultimately leading to a measurable change in ID [5] [13]. This approach is useful for creating broad-spectrum metabolite sensors.
The following diagram illustrates the workflow for developing and operating a functionalized OECT biosensor.
The Scientist's Toolkit: Research Reagent Solutions
The table below details essential materials and their functions for constructing and functionalizing OECT-based biosensors, as derived from recent literature.
Table 1: Key Research Reagents for OECT Biosensor Fabrication
| Item Name | Function/Description | Key Application in OECT Biosensing |
|---|---|---|
| PEDOT:PSS | Benchmark p-type OMIEC; high conductivity and biocompatibility [13]. | Standard channel material; can be chemically tuned for enhanced performance or specific sensing [5] [13]. |
| Gold (Au) Gate Electrodes | Polarizable electrode; easily functionalized with thiolated biomolecules [5]. | Platform for immobilizing enzymes, antibodies, or DNA probes for specific analyte recognition [5] [24]. |
| Ag/AgCl Gate Electrodes | Non-polarizable electrode with stable potential; enables smaller device footprints [5]. | Used as a stable reference, often in ion-sensing or when a constant gate potential is critical [5]. |
| Phosphate Buffered Saline (PBS) | Standard aqueous electrolyte with physiological pH and ion concentration. | Liquid electrolyte for in-vitro testing and characterization of OECT biosensor performance [13]. |
| Poly(ethylene glycol) (PEG)-based Hydrogels | Synthetic hydrogel electrolyte; tunable mechanical properties and ion conductivity [16]. | Solid-state electrolyte for wearable and implantable devices; reduces leakage and improves stability [16]. |
| Chitosan-based Hydrogels | Natural hydrogel derived from chitin; offers inherent biocompatibility [16]. | Biocompatible solid electrolyte for applications requiring close tissue integration [16]. |
| Glucose Oxidase (GOx) | Enzyme that catalyzes the oxidation of glucose to gluconolactone and H2O2 [5]. | Biorecognition element immobilized on the gate for OECT-based glucose biosensors [5] [24]. |
| Aptamers | Short, single-stranded DNA or RNA molecules that bind to a specific target. | Synthetic biorecognition elements for gate functionalization to detect proteins, ions, or small molecules [5]. |
| Valtropine | Valtropine, MF:C13H23NO2, MW:225.33 g/mol | Chemical Reagent |
| Mesutoclax | Mesutoclax, CAS:2760536-87-4, MF:C45H50ClN7O8S, MW:884.4 g/mol | Chemical Reagent |
Quantitative Performance Metrics of OECT Biosensors
The performance of OECT biosensors is quantitatively evaluated using several key metrics, including sensitivity, limit of detection (LOD), and dynamic range. The following table summarizes reported performance for various targets, highlighting the efficacy of different sensing mechanisms.
Table 2: Performance Metrics of OECT Biosensors for Various Analytes
| Target Analyte | Sensing Mechanism | Limit of Detection (LOD) | Sensitivity | Key Material/Strategy | Ref. |
|---|---|---|---|---|---|
| Glucose | Gate Functionalization (GOx) | ~100 nM | High (μA/mM) | Au gate with enzyme/redox mediator | [5] |
| Dopamine (DA) | Gate Functionalization | ~10 nM | -- | Selective membrane/functionalized gate | [5] |
| Lactate (LA) | Gate Functionalization / Electrolyte Functionalization | -- | -- | Lactate oxidase enzyme | [5] |
| DNA | Gate Functionalization (Aptamer) | -- | -- | Capacitive change from target binding | [5] |
| Proteins (e.g., IgG) | Gate Functionalization (Antibody) | -- | -- | Binding-induced capacitive or potential shift | [5] |
| Ions (Na+, K+) | Channel Functionalization | -- | -- | Ion-selective channel or membrane | [5] [16] |
Detailed Experimental Protocol: OECT-based Glucose Biosensing
This protocol provides a step-by-step methodology for fabricating and characterizing a gate-functionalized OECT for glucose detection, a canonical application in the field.
Device Fabrication
- Substrate Preparation: Clean a glass or flexible PET substrate sequentially with acetone, isopropanol, and deionized water in an ultrasonic bath for 10 minutes each. Dry under a stream of nitrogen.
- Electrode Patterning: Using photolithography or shadow masking, pattern and deposit a 5 nm Cr adhesion layer followed by a 50 nm Au layer to form the source and drain electrodes (channel length L = 10-100 µm, width W = 100-1000 µm).
- Channel Deposition: Spin-coat a commercially available PEDOT:PSS solution (e.g., Clevios PH 1000) onto the substrate at 2000 rpm for 60 seconds, covering the gap between the source and drain. Anneal on a hotplate at 120°C for 15 minutes to dry the film.
- Gate Electrode Fabrication: Pattern a separate Au gate electrode on a separate substrate or integrate it on the same chip.
Gate Functionalization
- Gate Cleaning: Clean the Au gate electrode via oxygen plasma treatment for 2 minutes.
- Self-Assembled Monolayer (SAM) Formation: Immerse the gate in a 1 mM solution of 6-mercapto-1-hexanol (MCH) in ethanol for 1 hour. This forms a SAM that passivates the surface and provides a base for enzyme attachment.
- Enzyme Immobilization: Incubate the SAM-modified gate with a solution containing 10 mg/mL Glucose Oxidase (GOx) and 5 mM 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) in a phosphate buffer (pH 7.4) for 2 hours at 4°C. EDC catalyzes the formation of amide bonds between carboxyl groups on the enzyme and the terminal hydroxyl groups of the MCH SAM.
- Rinsing and Storage: Rinse the functionalized gate thoroughly with phosphate buffer to remove unbound enzyme. Store in PBS at 4°C until use.
Electrical Characterization and Sensing
- Setup Assembly: Assemble the OECT in an electrochemical cell filled with PBS (0.1 M, pH 7.4) as the electrolyte. Connect the source, drain, and gate to a source measure unit (e.g., Keithley 2600B) or a potentiostat.
- Baseline Characterization: In the absence of glucose, measure the output characteristics (ID vs. VD at different VG) and transfer characteristics (ID vs. VG at a fixed VD, e.g., -0.1 V to -0.5 V). Calculate the transconductance (gm) from the transfer curve.
- Glucose Sensing:
- Apply a constant VD (e.g., -0.3 V) and a constant VG (e.g., 0.5 V for an Au gate).
- Monitor the drain current (ID) in real-time.
- Sequentially add small volumes of concentrated glucose stock solution to the electrolyte to achieve desired final concentrations (e.g., from 1 µM to 10 mM).
- Allow the current to stabilize after each addition.
- Data Analysis:
- Plot the change in ID (ΔID) or normalized current change (ΔID/ID(initial)) as a function of glucose concentration.
- Fit the data to a suitable model (e.g., Michaelis-Menten kinetics for enzymatic sensors) to extract the sensitivity and LOD (typically calculated as 3× the standard deviation of the baseline noise divided by the sensitivity).
OECTs represent a versatile and powerful tool for transducing biological events into quantifiable electrical signals. Their core mechanisms—gate, channel, and electrolyte functionalization—leverage the interplay between ionic and electronic charges in OMIECs to provide highly sensitive and amplified biosensing capabilities. As research progresses, the integration of OECTs with advanced materials like gel electrolytes [16] and their incorporation into closed-loop systems such as implantable drug delivery platforms [24] promise to unlock new frontiers in personalized medicine, real-time health monitoring, and diagnostic technologies. The protocols and data outlined herein provide a foundational framework for researchers and scientists to advance this rapidly evolving field.
Fabrication and Functionalization: Methodologies for Diverse Biosensing Applications
The integration of organic electrochemical transistors (OECTs) into advanced biosensing platforms requires fabrication methodologies that support mechanical flexibility, biocompatibility, and scalable production. This document details application notes and protocols for leveraging flexible printed circuit board (fPCB) technology and customizable inkjet printing to create high-performance, scalable OECT biosensors. These techniques enable the development of wearable and implantable devices capable of precise biomarker detection for research and clinical applications, combining the mechanical advantages of fPCBs with the patterning precision of advanced printing techniques [5] [25] [26].
fPCB Technology for OECT Biosensing Platforms
Material Selection and Stack-Up Design
The foundation of a reliable OECT-fPCB platform lies in the appropriate selection of materials that ensure both electrical performance and mechanical durability.
- Substrate and Copper: Polyimide is the standard substrate material due to its excellent thermal stability (withstanding soldering temperatures up to 260°C), mechanical flexibility, and low dielectric constant (Dk ≈ 3.2 @ 1GHz), which reduces signal loss [27] [28] [29]. For the conductive layers, rolled annealed copper is preferred over electrodeposited copper for dynamic flexing applications, as it offers superior ductility and resistance to fatigue cracking during repeated bending [27] [28].
- Stack-Up Configuration: A typical 2-layer fPCB stack-up for an OECT biosensor might consist of a 25μm polyimide core, with 18μm rolled annealed copper layers on both sides, laminated using adhesives or adhesiveless processes. Adhesiveless laminates are recommended for enhanced reliability as they prevent delamination under mechanical stress [27] [28]. The stack-up must be designed to balance flexibility with the need for signal and ground planes for impedance control.
Table 1: fPCB Material Properties for OECT Integration
| Material/Property | Polyimide (Flex) | FR-4 (Rigid for Stiffeners) | Impact on OECT Biosensor Design |
|---|---|---|---|
| Dielectric Constant (Dk) @1GHz | ~3.2 [28] | ~4.5 [28] | Lower signal loss for high-frequency biosensing signals |
| Glass Transition Temp (Tg) | >250°C [28] | 130-180°C [28] | Withstands high-temperature processing and soldering |
| Coefficient of Thermal Expansion (CTE) | 12-15 ppm/°C [28] | 14-18 ppm/°C [28] | Reduced warping and better compatibility with coated OMIECs |
| Moisture Absorption | 2.8% [28] | 0.8% [28] | Requires pre-bake before assembly to prevent outgassing |
Critical fPCB Layout and Routing Guidelines
OECT biosensors require meticulous layout to maintain integrity during both static integration and dynamic operation in wearable formats.
- Bend Radius Management: Violations of the minimum bend radius account for a majority of field failures in flexible circuits [28]. Adherence to IPC-2223 standards is critical. For a static bend (e.g., a one-time fit into a device housing), the minimum radius is typically 10x the total board thickness. For dynamic bends (e.g., in a joint monitor), the minimum radius should be 100-150x the thickness [28] [29]. For example, a 0.15mm thick single-layer flex should have a dynamic bend radius no smaller than 15mm.
- Trace Routing and Via Placement: Conductors in bend areas should be routed perpendicular to the bend axis to minimize stress [28]. Sharp 90° angles should be avoided in favor of curved traces [27] [29]. Plated through-hole (PTH) vias are rigid and must be kept out of bend zones; a minimum distance of 3x the board thickness from the bend line is recommended. Teardrop pads should be used at trace-to-via junctions to reduce stress concentration [27] [28].
- Neutral Axis Optimization: To prevent tensile or compressive forces on copper traces during bending, critical traces should be positioned near the mechanical neutral axis of the flex stack-up. This can be achieved through careful layer planning, placing thin signal traces between layers of polyimide [28].
- Stiffener Integration: Components like microcontrollers or passive components require rigid support. Stiffeners made of FR4 or polyimide should be laminated to the fPCB in areas with component placements, ensuring they do not impinge on designated flex zones [27] [29].
Table 2: fPCB Bend Radius Guidelines per IPC-2223
| Flex Layer Count | Total Thickness (mm) | Static Min Bend Radius | Dynamic Min Bend Radius |
|---|---|---|---|
| Single Layer | 0.15 | 1.5mm (10:1 ratio) [28] | 15mm (100:1 ratio) [28] |
| Double Layer | 0.25 | 2.5mm (10:1 ratio) [28] | 37.5mm (150:1 ratio) [28] |
| Multi-Layer (4+) | 0.50 | 10mm (20:1 ratio) [28] | Not Recommended [28] |
Protocol: fPCB Design and Fabrication for a Wearable OECT Biosensor
Objective: To design and fabricate a 2-layer fPCB that serves as the substrate and interconnect for a dynamic flexing OECT-based lactate sensor integrated into athletic wear.
Materials:
- Polyimide substrate (25μm)
- Rolled annealed copper foil (18μm, 0.5 oz)
- Adhesiveless laminate material
- Polyimide-based coverlay (25μm)
- FR4 stiffener (0.2mm) for component areas
Procedure:
- Define Bend Requirements: Identify the specific area of the textile that will experience repeated bending during movement. Classify this as a dynamic flex zone.
- Calculate Minimum Bend Radius: Based on a 2-layer stack-up with an estimated final thickness of 0.2mm, the dynamic bend radius is calculated as 0.2mm x 150 = 30mm [28]. Incorporate a 20% safety margin, resulting in a design radius of 36mm.
- Stack-Up Design: Finalize a stack-up: Top Coverlay / Signal Layer 1 (Cu) / Polyimide Core / Signal Layer 2 (Cu) / Bottom Coverlay.
- CAD Layout: a. Component Placement: Place all integrated circuits (ICs) and connectors in rigid sections defined by FR4 stiffeners. b. Trace Routing: In the dynamic flex zone, route traces carrying signals from the OECT perpendicular to the bend axis. Use curved traces with a minimum radius of 0.5mm [29]. Maintain a trace/space of at least 4/4mil (0.1mm) [29]. c. Via and Pad Design: Do not place vias within 0.6mm (3 x 0.2mm) of the bend line. Apply teardrops to all pads connected to traces.
- Design Rule Check (DRC): Run a DRC using the manufacturer's specific constraints for flex circuits, verifying spacing, annular rings, and bend zone rules.
- Panelization and Fabrication: Panelize the design with adequate spacing (≥2mm) and submit the Gerber files to a manufacturer with IPC-6013 Class 3 certification for flexible circuits [28] [29].
Customizable Inkjet Printing for OECT Fabrication
High-Resolution Micro-Dispensing Technology
Inkjet printing has evolved into a powerful additive manufacturing technique for depositing functional materials in OECTs. Recent advancements in high-resolution micro-dispensing enable the monolithic integration of all OECT components with exceptional precision.
- Technology Overview: Modern micro-dispensing systems can handle a wide viscosity range of inks (10 - 10âµ cP) and offer femtoliter-volume control with micrometer-scale resolution [26]. This allows for the precise deposition of conductors, semiconductors, insulators, and even gel electrolytes directly onto flexible substrates, including the fPCBs described in Section 2.
- Performance Capabilities: This technique has been used to fabricate fully printed OECTs with record intrinsic gains of 330 V/V and OECT-based amplifier circuits with a gain-bandwidth product of 1 MHz, the highest reported for fully printed OECTs [26]. This performance is critical for amplifying weak biosignals in real-time.
Functional Inks for OECT Biosensors
The efficacy of inkjet-printed OECTs hinges on the formulation of functional inks.
- Channel Materials: The most common organic mixed ionic-electronic conductor (OMIEC) is PEDOT:PSS. Inks are formulated with additives like surfactants and secondary dopants (e.g., ethylene glycol) to optimize jetting stability, film formation, and ultimately, electrical conductivity and ion transport [5] [6].
- Gate Electrodes: For biosensing, non-polarizable gate electrodes such as Ag/AgCl are often preferred. Silver silver-chloride (Ag/AgCl) inks can be printed to create stable reference gates [5] [6]. Polarizable gates made from gold or platinum nanoparticle inks are also used.
- Electrolytes: Hydrogel-based inks can be formulated to create solid-state or quasi-solid-state electrolyte layers that are compatible with printing and suitable for wearable applications [12] [26].
Protocol: Inkjet Printing of an OECT for Glucose Sensing
Objective: To fabricate a gate-functionalized OECT for glucose detection on a polyimide fPCB substrate using inkjet printing.
Materials:
- Pre-fabricated fPCB with Au source/drain electrodes
- PEDOT:PSS ink formulation (with 5% ethylene glycol dopant)
- Ag/AgCl nanoparticle ink
- Glucose oxidase (GOx) enzyme solution
- Nafion ionomer solution
- Insulating dielectric ink
Procedure:
- Substrate Preparation: Clean the fPCB substrate with oxygen plasma to ensure a hydrophilic surface for optimal ink adhesion.
- Print OECT Channel: a. Load the PEDOT:PSS ink into a piezoelectric printhead. b. Using a waveform optimized for the ink's viscosity and surface tension, print the OECT channel layer, defining a precise geometry (e.g., W=1000μm, L=100μm) between the pre-existing source and drain contacts on the fPCB. c. Thermally cure the film at 120°C for 15 minutes in a nitrogen environment.
- Print and Functionalize Gate Electrode: a. Print the Ag/AgCl gate electrode structure in a dedicated area on the fPCB. b. Cure the gate electrode at 80°C for 30 minutes. c. Gate Functionalization: Deposit a mixed ink containing GOx and Nafion directly onto the printed gate electrode. The Nafion acts as a permselective membrane. Air-dry the functionalization layer at room temperature.
- Define Electrolyte Cavity: Print a dam wall of insulating dielectric ink around the OECT channel and gate to contain the liquid electrolyte.
- Curing and Validation: Perform a final low-temperature cure (60°C for 1 hour) to stabilize all layers without damaging the biological component (GOx). Validate device performance by measuring transfer and output characteristics in a phosphate buffer solution.
Integrated Workflow and Biosensing Application
The synergy between fPCB technology and inkjet printing enables a seamless workflow for creating advanced biosensing systems. The following diagram illustrates the integrated fabrication and sensing process.
Diagram 1: Integrated fPCB and Inkjet Printing Workflow for OECT Biosensors. This diagram outlines the sequential process from fPCB substrate fabrication to OECT printing/functionalization and final biosensing operation.
Biosensing Mechanism and Experimental Protocol
The operational principle of the integrated biosensor relies on the modulation of the OECT's channel current by a biochemical reaction at the functionalized gate.
Biosensing Mechanism: In a typical glucose sensor, the mechanism follows a gate-functionalization approach [5] [6]:
- Glucose in the analyte solution diffuses to the functionalized gate electrode.
- The immobilized glucose oxidase (GOx) enzyme catalyzes the oxidation of glucose to gluconolactone, consuming oxygen and generating hydrogen peroxide (Hâ‚‚Oâ‚‚).
- The subsequent electrochemical oxidation of Hâ‚‚Oâ‚‚ at the gate electrode surface (e.g., Ag/AgCl) generates a Faradaic current or alters the effective gate potential (VGeff).
- This change in VGeff modulates the conductivity of the PEDOT:PSS channel, leading to a measurable change in the drain current (ID). The magnitude of this change is proportional to the concentration of glucose [5].
Diagram 2: OECT Biosensing Mechanism via Gate Functionalization. This diagram visualizes the cascade from analyte-enzyme interaction to the final electrical signal readout.
Experimental Protocol: Lactate Sensing in Simulated Sweat
Objective: To characterize the performance of a printed OECT biosensor on fPCB for detecting lactate in an artificial sweat solution.
Materials:
- fPCB-integrated OECT with Lactate Oxidase (LOx)-functionalized gate
- Artificial sweat solution (pH ~5.5)
- Lactate standard solutions (0.1mM, 0.5mM, 1.0mM, 5.0mM)
- Potentiostat/Galvanostat or custom readout circuit
- Phosphate Buffered Saline (PBS, 1X) for washing
Procedure:
- Baseline Measurement: Place the OECT sensor in a well containing artificial sweat solution without lactate. Apply a constant drain voltage (VD = -0.3 V) and record the steady-state drain current (ID) while sweeping the gate voltage (VG) from 0 to 0.5 V to obtain the baseline transfer curve [5] [6].
- Sensitivity and Calibration: a. Replace the solution with 0.1mM lactate in artificial sweat. b. At a fixed VG within the device's sensitive range (e.g., 0.3 V), record the change in ID over time until a new steady state is reached. c. Rinse the sensor with PBS and repeat step 2b for each increasing concentration of lactate standard. d. Plot the steady-state ΔID (or normalized ID/ I0) versus lactate concentration. Fit the data to a linear or Michaelis-Menten model to establish a calibration curve.
- Selectivity Test: To verify specificity, expose the sensor to potential interferents in artificial sweat (e.g., glucose, urea, ascorbic acid) at physiologically relevant concentrations and measure the device response. A well-designed sensor with a permselective membrane (e.g., Nafion) should show minimal response to interferents.
- Stability Test: Perform a continuous measurement of the sensor's response to a fixed lactate concentration over several hours to assess operational stability.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Materials for fPCB-based, Inkjet-Printed OECT Biosensors
| Item | Function/Description | Research Application Note |
|---|---|---|
| Polyimide Substrate | Flexible, thermally stable base material for the fPCB. | Provides mechanical backbone; choose 25-50μm thickness for optimal flexibility vs. handling [27] [29]. |
| Rolled Annealed Copper | Conductive traces for interconnects and electrodes. | Superior for dynamic flexing; use 0.5-1 oz thickness [28]. |
| PEDOT:PSS Ink | Organic semiconductor for the OECT channel. | The workhorse OMIEC; requires formulation with secondary dopants (e.g., DMSO, EG) for high conductivity and printability [5] [6] [26]. |
| Ag/AgCl Ink | Material for printing stable, non-polarizable reference gate electrodes. | Essential for stable potentiometric sensing and minimizing gate voltage drift [5] [6]. |
| Glucose Oxidase (GOx) | Recognition enzyme for glucose biosensing. | Immobilize on the gate electrode via cross-linking or entrapment in a polymer matrix like Nafion [5]. |
| Nafion | Ionomer membrane. | Coated on the gate to provide charge selectivity, repelling interferents like ascorbate and urate [5] [6]. |
| Polyimide Coverlay | Flexible insulating layer replacing solder mask. | Protects copper traces from corrosion and physical damage in flex zones; ensures durability [28] [29]. |
| Carmichaenine B | Carmichaenine B, MF:C23H37NO7, MW:439.5 g/mol | Chemical Reagent |
| 3-Acetylyunaconitine | 3-Acetylyunaconitine, MF:C37H51NO12, MW:701.8 g/mol | Chemical Reagent |
In the field of organic electrochemical transistor (OECT) biosensing, surface functionalization plays a pivotal role in determining device performance, particularly in terms of specificity, sensitivity, and stability. The gate electrode, which serves as the primary bio-recognition interface in OECTs, can be strategically modified to enhance biosensing capabilities for various applications, including drug development and pathogen detection [30]. This application note details advanced gate functionalization strategies, focusing on the use of N-heterocyclic carbenes (NHCs) as ultra-stable molecular anchors and their application in creating specific biosensing interfaces for drug probing. These protocols are presented within the context of ongoing OECT biosensing research, providing methodologies relevant to scientists and drug development professionals seeking to develop reliable, long-term biosensing platforms.
NHC Gate Functionalization: Principles and Advantages
N-heterocyclic carbenes have emerged as superior alternatives to traditional thiol-based chemistry for functionalizing metal gate electrodes in OECTs. Their exceptional stability stems from strong covalent bonds with transition metals like gold, characterized by a high dissociation energy of approximately 67 kcal/mol and a short bond length of 2.0 Ã… [31]. This results in functionalized surfaces that withstand thermal, hydrolytic, chemical, and oxidative stresses far better than thiol-based monolayers [31] [32].
The versatility of NHCs allows for their application on various conductive surfaces beyond gold, including platinum, palladium, copper, and even glassy carbon, significantly expanding their utility in electrode design [33] [34]. Furthermore, NHC layers can be engineered with specific functional termini (e.g., alkyne or azide groups) that serve as "clickable" platforms for subsequent covalent attachment of biorecognition elements via click chemistry, enabling highly specific biosensor development [33] [34].
Quantitative Performance of NHC-Functionalized OECTs
Biosensing Performance and Long-Term Stability
Table 1: Performance Metrics of NHC-Functionalized OECTs for Biosensing
| Performance Parameter | Value | Experimental Context |
|---|---|---|
| Streptavidin Detection Signal | 193 ± 64 mV | Threshold voltage shift (ΔVT) for biotin-streptavidin binding [31] [35] |
| Non-specific Binding Control | 62 ± 41 mV | Threshold voltage shift (ΔVT) for Bovine Serum Albumin (BSA) [31] [35] |
| Signal-to-Noise Ratio | ~3:1 | Ratio of streptavidin signal to BSA signal [31] |
| Long-term Stability | 24 months | Functional performance after room temperature storage [31] [35] [32] |
| Aged Device Performance | 161 ± 30 mV | Streptavidin detection signal after 24-month storage [31] [35] |
Material and Reagent Solutions
Table 2: Essential Research Reagent Solutions for NHC-OECT Fabrication and Functionalization
| Reagent/Category | Specific Examples | Function in Protocol |
|---|---|---|
| NHC Precursors | Imidazole, 3-bromo-1-(trimethylsilyl)-1-propyne [31] | Synthesis of N-heterocyclic carbene ligands for surface anchoring |
| Gate Electrode Materials | Gold nanoparticles (Au NP ink) [31] | Forms the functionalizable gate electrode surface |
| Channel Materials | PEDOT:PSS (Clevios PH 1000) [31] [23] | Conductive polymer channel for ion-to-electron transduction |
| Cross-linkers | (3-glycidyloxypropyl)trimethoxysilane (GOPS) [31] [23] | Enhances structural stability of polymer channels |
| Click Chemistry Components | Alkyne/azide-functionalized NHCs, Cu(I) catalyst [33] [34] | Covalent attachment of specific biorecognition elements |
| Biorecognition Elements | Biotin, antibodies, aptamers [31] [30] | Provides specific binding to target analytes |
Experimental Protocols
Protocol 1: Fabrication of Aerosol Jet-Printed OECTs
Principle: OECTs are fabricated on flexible substrates using aerosol jet printing, which enables precise deposition of electrode and channel materials, forming the foundational biosensor structure [31].
Materials: Polyimide substrate, gold nanoparticle (Au NP) ink (UT Dots, Inc.), PEDOT:PSS mixture (94% Clevios PH 1000, 5% ethylene glycol, 0.1% DBSA, 1% GOPS), UV-curable PDMS.
Procedure:
- Substrate Preparation: Clean polyimide substrate with isopropanol and dry under nitrogen stream.
- Electrode Printing: Print Au gate, source, and drain electrodes using ultrasonic atomizer with Au NP ink.
- Annealing Process: Anneal printed Au traces at 280°C for 1 hour on a hotplate.
- Channel Deposition: Print PEDOT:PSS channel mixture using ultrasonic atomizer onto defined channel area.
- Channel Curing: Anneal device in oven at 130°C for 20 minutes to cross-link PEDOT:PSS with GOPS.
- Insulation Layer: Deposit UV-curable PDMS layer diluted with hexanes (3:1 volume ratio) using pneumatic atomizer to protect metal traces.
- Curing: Cure PDMS layer with UV light during printing, followed by thermal annealing at 130°C for 30 minutes.
Protocol 2: NHC Functionalization of Au Gate Electrodes
Principle: N-heterocyclic carbene ligands form stable covalent bonds with gold surfaces, creating a robust monolayer for subsequent biomolecule immobilization [31] [33].
Materials: NHC ligand (synthesized according to Johnson et al. [31]), anhydrous acetonitrile, nitrogen environment setup, biotin-azide.
Procedure:
- NHC Synthesis:
- Perform under nitrogen environment using Schlenk technique.
- Dissolve 500 mg imidazole in 50 mL anhydrous acetonitrile.
- Add 2.42 mL 3-bromo-1-(trimethylsilyl)-1-propyne to imidazole solution.
- React for 24 hours at room temperature with stirring.
- Purify resulting NHC ligand by precipitation and washing.
Gate Electrode Functionalization:
- Clean Au gate electrodes with oxygen plasma treatment for 5 minutes.
- Immerse electrodes in 1 mM NHC solution in anhydrous acetonitrile for 12 hours at room temperature under nitrogen atmosphere.
- Rinse functionalized electrodes thoroughly with acetonitrile and ethanol to remove physisorbed ligands.
- Dry under nitrogen stream.
Biotin Immobilization (via Click Chemistry):
- Prepare biotin-azide solution (1 mM in PBS buffer).
- Apply solution to NHC-functionalized Au gate electrode.
- Catalyze using copper-catalyzed azide-alkyne cycloaddition (CuAAC) with 0.1 mM Cu(II) and sodium ascorbate as reducing agent.
- Incubate for 2 hours at room temperature.
- Rinse thoroughly with PBS buffer to remove unbound biotin.
Protocol 3: OECT Biosensing Measurements for Drug Probe Specificity
Principle: Functionalized OECTs detect binding events through threshold voltage shifts resulting from changes in gate potential upon analyte binding, providing quantitative assessment of drug-target interactions [31] [36].
Materials: Functionalized OECT devices, phosphate buffered saline (PBS, pH 7.4), target analytes (e.g., streptavidin, drug compounds), semiconductor parameter analyzer (e.g., Keithley 4200).
Procedure:
- Device Setup:
- Place OECT in electrochemical cell with gate electrode immersed in PBS.
- Connect source, drain, and gate electrodes to semiconductor parameter analyzer.
Baseline Measurement:
- Apply drain-source voltage (VDS) of -0.5 V.
- Sweep gate voltage (VGS) from 0.2 V to -0.6 V.
- Record transfer characteristics (IDS vs. VGS).
- Extract baseline threshold voltage (VT) from transfer curve.
Analyte Detection:
- Introduce target analyte (e.g., streptavidin at various concentrations) to electrolyte solution.
- Allow binding reaction to proceed for 10-15 minutes.
- Measure transfer characteristics after binding equilibrium established.
- Calculate threshold voltage shift (ΔVT) relative to baseline.
Specificity Assessment:
- Repeat measurement with non-target proteins (e.g., BSA) to assess nonspecific binding.
- Compare ΔVT values between target and non-target analytes.
- Calculate signal-to-noise ratio as ΔVT,target/ΔVT,non-target.
Implementation Workflow and OECT Operational Mechanism
NHC-OECT Fabrication and Biosensing Workflow - This diagram outlines the comprehensive process from device fabrication to functionalization and biosensing application, highlighting the key stages in developing NHC-functionalized OECT biosensors.
OECT Biosensing Mechanism with NHC-Functionalized Gate - This diagram illustrates the signal transduction mechanism in NHC-functionalized OECTs, showing how target binding at the gate interface is amplified into measurable electrical signals.
The integration of N-heterocyclic carbene chemistry with OECT technology represents a significant advancement in biosensor design, particularly for applications requiring long-term stability and high specificity. The protocols outlined herein provide researchers with robust methodologies for developing reliable biosensing platforms capable of detecting specific biological interactions with minimal nonspecific binding. The exceptional 24-month stability demonstrated by NHC-functionalized OECTs [31] [35], combined with the versatility of click chemistry for attaching various biorecognition elements, positions this approach as a valuable tool for drug discovery, diagnostic development, and fundamental biological research. As the field progresses, the integration of these functionalization strategies with emerging materials such as gel electrolytes [37] and porous channel architectures [23] will further expand the capabilities of OECT-based biosensing platforms.
Channel and Electrolyte Interface Engineering for Enhanced Sensing
The performance of organic electrochemical transistor (OECT)-based biosensors is fundamentally governed by the intricate interface between the organic semiconductor channel and the electrolyte. Engineering this interface is critical for enhancing key sensor metrics, including sensitivity, detection limit, stability, and specificity [5] [16]. OECTs operate through the reversible injection of ions from the electrolyte into the bulk of the channel material, modulating its electronic conductivity [5]. This mechanism provides high transconductance and significant signal amplification, making OECTs exceptionally suitable for detecting biological signals in aqueous environments [38] [39]. This document outlines application notes and detailed protocols for engineering the channel and electrolyte interfaces, providing a practical guide for researchers and scientists working on advanced biosensing platforms within the broader context of OECT research.
Channel Engineering Strategies
The composition and structure of the OECT channel are primary determinants of device performance. Channel engineering focuses on optimizing the organic mixed ionic-electronic conductor (OMIEC) to achieve high charge carrier mobility (μ) and large volumetric capacitance (C), collectively represented by the figure of merit μC [38] [16].
Material Selection and Blending
Application Note: Blending conjugated polymers with non-conjugated radical polymers presents a powerful strategy to precisely control a channel's electrochemical and ionic transport properties [38]. The conjugated polymer provides the pathway for electronic charge transport, while the radical polymer facilitates and modulates ion injection.
Protocol: Fabrication of a Blended Polymer Channel for Dopamine Sensing
- Objective: To create a high-performance OECT channel with a μC* > 190 F Vâ»Â¹ cmâ»Â¹ sâ»Â¹ for ultrasensitive dopamine detection.
- Materials:
- Conjugated Polymer: Poly(3-(methoxyethoxyethoxy)thiophene) (P3MEET) [38].
- Radical Polymers: Poly(4-episulfide-2,2,6,6-tetramethylpiperidine-1-oxyl) (PTES) or Poly(4-glycidyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl) (PTEO) [38].
- Solvents: Anhydrous Chlorobenzene for P3MEET; appropriate solvents for radical polymers (e.g., DMF).
- Procedure:
- Polymer Synthesis: Synthesize P3MEET via Grignard Metathesis (GRIM) polymerization. Synthesize PTES and PTEO via anionic ring-opening polymerization (AROP) of their respective monomers to achieve high radical densities (>99%) [38].
- Solution Preparation: Prepare separate stock solutions of P3MEET, PTES, and PTEO.
- Blending: Mix the P3MEET solution with the PTES or PTEO solution to achieve the desired weight ratio (e.g., 1:1 for 50 wt% radical polymer). Vortex and agitate the mixture thoroughly to ensure homogeneity [38].
- Film Deposition: Spin-coat the blended solution onto pre-patterned source-drain electrodes (e.g., Gold on PEN or glass).
- Post-processing: Anneal the film as required (e.g., at 120°C for 1 hour) to remove residual solvents and enhance stability [38].
Table 1: Performance of Engineered OECT Channels
| Channel Material/Strategy | Key Performance Metric (μC*) | Target Analyte | Reported Detection Limit | Key Advantage |
|---|---|---|---|---|
| P3MEET:PTES (50:50 wt%) [38] | 192 F Vâ»Â¹ cmâ»Â¹ sâ»Â¹ | Dopamine | 1 pM | Precise control of ion injection, high sensitivity |
| Porous PEDOT:PSS (Freeze-Dried) [23] | Reduced vs. film (increased effective L) | Cations (Kâº), Hâ‚‚Oâ‚‚ | N/A | 3D scaffold for cell culture, mimics tissue |
| n-type CPE-Aptamer Hybrid [40] | N/A | Dopamine | Attomolar (aM) | High specificity, covalent bioreceptor attachment |
| PEDOT:PSS [16] | Up to 1500 F cmâ»Â¹ Vâ»Â¹ sâ»Â¹ | General OECT operation | N/A | High conductivity, industry standard |
Structural and Chemical Modification
Application Note: Creating porous or three-dimensional channel architectures significantly increases the interfacial surface area between the channel and the electrolyte. This enhances ion uptake and can transform the channel into a bioactive scaffold, enabling direct monitoring of 3D cell cultures [23].
Protocol: Fabrication of a Porous PEDOT:PSS Channel via Freeze-Drying
- Objective: To create a macroporous OECT channel that functions as an electroactive scaffold for 3D cell cultures.
- Materials: PEDOT:PSS dispersion (e.g., Orgacon ICP 1050), (3-glycidyloxypropyl)trimethoxysilane (GOPS) cross-linker [23].
- Procedure:
- Cocktail Preparation: Prepare a mixture of 1.0 wt% PEDOT:PSS and 3.0 wt% GOPS in an aqueous solution [23].
- Deposition: Drop-cast 5 µL of the PEDOT:PSS/GOPS cocktail onto the predefined channel area between the source and drain electrodes.
- Freezing: Immediately transfer the device to a -80 °C freezer and freeze for 1 hour.
- Freeze-Drying: Place the frozen device in a freeze-dryer to sublimate the ice crystals, leaving behind a porous polymer network.
- Cross-Linking: Anneal the device at 120 °C for 1 hour to thermally cross-link GOPS, stabilizing the porous structure [23].
- Characterization: Use Scanning Electron Microscopy (SEM) to confirm pore formation and size distribution (typical median diameter ~50 µm) [23].
Electrolyte Engineering Strategies
The electrolyte is the medium through which biological signals are transduced into ionic currents. Replacing liquid electrolytes with solid-state gels mitigates issues of leakage and evaporation, enabling robust, wearable, and implantable devices [16].
Gel Electrolyte Integration
Application Note: Gel electrolytes, including hydrogels and ionic liquid (IL) gels, combine the high ionic conductivity of liquids with the mechanical stability and safety of solids. Their soft, hydratable nature ensures conformal contact with biological tissues and reduces interfacial impedance [16].
Protocol: Formulating and Integrating a PVA-Based Hydrogel Electrolyte
- Objective: To create a stable, solid-state OECT with a hydrogel electrolyte for operation in flexible or wearable form factors.
- Materials: Poly(Vinyl Alcohol) (PVA), Phosphate Buffered Saline (PBS, 1X) or Sodium Chloride (NaCl), deionized water, mold (e.g., Petri dish) [16].
- Procedure:
- Polymer Solution: Dissolve PVA granules in deionized water (e.g., 10% w/v) at 90°C with vigorous stirring until the solution is clear.
- Salination: Add a concentrated salt solution (e.g., PBS or NaCl) to the warm PVA solution to achieve the desired ionic strength (e.g., 0.1 M). Mix thoroughly.
- Gelation: Pour the mixture into a mold and allow it to cool to room temperature to form a physical gel. For stronger gels, perform freeze-thaw cycles.
- Integration: Cut the hydrogel into an appropriate-sized piece and place it directly onto the OECT, ensuring it bridges the channel and the gate electrode [16].
- Encapsulation: Gently encapsulate the device to hold the hydrogel in place without compressing it, ensuring stable contact.
Table 2: Comparison of Gel Electrolytes for Solid-State OECTs
| Gel Electrolyte Type | Example Materials | Key Properties | Best Suited Applications |
|---|---|---|---|
| Synthetic Hydrogels [16] | PVA, PHEA, PAAm | Tunable mechanics, high hydration, biocompatibility | Wearable sensors, skin-contact devices |
| Natural Hydrogels [16] | Chitosan, Gelatin, Agar | Inherent biocompatibility, low cytotoxicity | Short-term implantable sensors |
| Composite Hydrogels [16] | PAAm-Carrageenan network | Enhanced mechanical strength, anti-freeze properties | Harsh environments, low-temperature operation |
| Ionic Liquid (IL) Gels [16] | [EMIM][TFSI] in polymer matrix | High thermal stability, non-volatile, high conductivity | Flexible electronics requiring wide operational windows |
Interface Functionalization and Sensing Mechanisms
Functionalizing the channel or gate interface with biorecognition elements is essential for imparting high specificity to the OECT biosensor [5] [41].
Gate and Channel Functionalization
Application Note: The most common biosensing strategy involves immobilizing bioreceptors (e.g., antibodies, aptamers) on the gate electrode. Binding of the target analyte alters the effective gate potential, modulating the channel current [5] [41]. Direct functionalization of the channel is an alternative, where analyte binding changes the channel's electronic properties [5].
Protocol: Carboxylic Acid-Based Gate Functionalization for Antibody Immobilization
- Objective: To create a functionalized gate electrode for specific detection of human Immunoglobulin G (IgG).
- Materials: ITO/PET substrate, PT-COOH polymer (or PSAA, or DDA for SAM), EDC/NHS crosslinking kit, Ethanol, Human IgG antibody (receptor), Ethanolamine (for blocking) [41].
- Procedure:
- Substrate Cleaning: Clean the ITO gate electrode with isopropanol and treat with UV-Ozone for 30 minutes [41].
- Receptor Layer Deposition:
- For Polymer (PT-COOH/PSAA): Spin-coat the polymer solution onto the ITO gate and anneal.
- For SAM (DDA): Immerse the ITO gate in an ethanol solution of 1,10-decanedicarboxylic acid (DDA) to form a self-assembled monolayer [41].
- Activation: Incubate the functionalized gate with a mixture of EDC and NHS in water to activate the carboxylic acid groups.
- Antibody Immobilization: Incubate the activated gate with a solution of human IgG antibody. The antibody's amine groups will form covalent amide bonds with the activated surface.
- Blocking: Incubate the gate with ethanolamine to deactivate any remaining activated esters and prevent non-specific binding.
- Measurement: Integrate the functionalized gate into the OECT setup and characterize using transfer curves or real-time current monitoring [41].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for OECT Interface Engineering
| Reagent/Material | Function/Application | Example Usage & Rationale |
|---|---|---|
| PEDOT:PSS (PH1000) [16] [42] | p-type OECT channel material | High μC*, industry standard, used for electrophysiology and general sensing. |
| P3MEET [38] | EGylated conjugated polymer | Channel material in blends; enhances ion transport and carrier mobility. |
| PTES / PTEO [38] | Radical polymers with TEMPO groups | Blended with conjugated polymers to regulate ion injection and dope levels. |
| GOPS [23] [42] | Cross-linker | Stabilizes polymer films (e.g., PEDOT:PSS) against dissolution in aqueous media. |
| n-type CPEs [40] | n-type OECT channel material | Enables creation of complementary circuits; can be functionalized with aptamers. |
| PVA / Chitosan [16] | Hydrogel matrix | Forms solid-state gel electrolytes for wearable and implantable devices. |
| EDC / NHS [41] | Cross-linking chemistry | Activates carboxyl groups for covalent immobilization of biomolecules on surfaces. |
| Aptamers [40] | Biorecognition element | Provides high specificity; can be covalently attached to functionalized channels/gates. |
| Ykl-5-124 | Ykl-5-124, MF:C28H33N7O3, MW:515.6 g/mol | Chemical Reagent |
| Sessilifoline A | Sessilifoline A, MF:C22H31NO5, MW:389.5 g/mol | Chemical Reagent |
Visualizing OECT Biosensing Mechanisms and Workflows
The following diagrams illustrate the core sensing mechanisms and a generalized experimental workflow for OECT biosensor development.
OECT Biosensing Mechanisms
OECT Biosensor Fabrication and Testing Workflow
Organic Electrochemical Transistors (OECTs) have emerged as a transformative platform in biosensing, particularly for the detection of small molecules critical to physiological functions. Their high transconductance, low operating voltage, and excellent biocompatibility make them ideally suited for monitoring metabolites like glucose, dopamine, and lactate in complex biological environments [5]. These devices transduce biochemical interactions into amplified electrical signals, enabling highly sensitive and real-time detection of target analytes. This application note details the working principles, performance metrics, and standardized experimental protocols for OECT-based detection of these key small molecules, providing a framework for their application in biomedical research and drug development.
Working Principles of OECT Biosensors
An OECT is a three-terminal device consisting of a source, a drain, and a gate electrode. The channel between the source and drain is typically fabricated from an organic mixed ionic-electronic conductor (OMIEC), with poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) being the most prevalent [13]. The gate electrode is immersed in an electrolyte that is in contact with the channel. The fundamental operating mechanism involves the electrochemical doping and dedoping of the channel material via the injection or extraction of ions from the electrolyte, modulated by the gate voltage ((VG)) [5]. When a bias voltage ((VD)) is applied between the source and drain, the resulting drain current ((ID)) is controlled by (VG). The efficiency of this conversion is quantified by the transconductance ((gm = \partial ID / \partial V_G)), a key performance parameter for OECTs [13].
For biosensing applications, the primary mechanism involves functionalizing part of the OECT to be sensitive to a specific target analyte. The three main strategies are (1) Gate Functionalization, where the gate electrode is modified with a recognition element (e.g., an enzyme); (2) Channel-Electrolyte Interface Functionalization; and (3) Electrolyte Functionalization [5]. A change in the analyte concentration induces a variation in the effective gate potential or the channel's doping state, leading to a measurable shift in the transfer characteristics ((ID) vs. (VG)) or a change in (ID) at a fixed (VG) [5].
The diagram below illustrates the primary biosensing mechanisms and experimental workflow for OECT-based small molecule detection.
Performance Comparison of Small Molecule Sensors
The performance of OECT biosensors for detecting glucose, dopamine, and lactate is summarized in Table 1, highlighting their sensitivity, detection limits, and linear dynamic range.
Table 1: Performance Metrics of OECT-based Biosensors for Small Molecules
| Analyte | Recognition Element | Linear Detection Range | Detection Limit | Key Material/Strategy | Reference |
|---|---|---|---|---|---|
| Glucose | Glucose Oxidase (GOx) | Not fully specified in results | High sensitivity reported | Prussian Blue (PB) gate; NFC system for sweat | [43] |
| Dopamine | Aptamers | 0.1 nM – 10 nM (covers clinical range) | Femto- to pico-molar range | Aptamer receptors with OECTs | [44] |
| Lactate | Lactate Oxidase (LOx) | Up to ~1 mM | 11 nM | LOx immobilized with chitosan/glutaraldehyde on Pt/PB gate | [45] |
Experimental Protocols
Protocol: Fabrication of a Lactate OECT Biosensor
This protocol outlines the steps for fabricating and characterizing an OECT optimized for lactate sensing, based on the work by [45].
Research Reagent Solutions
| Item | Function/Brief Explanation |
|---|---|
| Flexible Kapton Substrate | Provides a robust, flexible base for the device. |
| Gold (Au) Source/Drain Electrodes | Inert conductors for electronic current injection/collection. |
| PEDOT:PSS Channel | OMIEC; transduces ionic signals from the electrolyte into electronic currents. |
| Prussian Blue (PB) Gate Electrode | Catalyzes the reduction of Hâ‚‚Oâ‚‚, enhancing sensitivity. |
| Lactate Oxidase (LOx) Enzyme | Biorecognition element; specifically catalyzes the oxidation of lactate. |
| Chitosan or Glutaraldehyde | Matrix for immobilizing and stabilizing the LOx enzyme on the gate. |
| Phosphate Buffer Saline (PBS) | Electrolyte for testing; provides a stable ionic environment. |
Procedure
- Device Fabrication: Pattern gold source, drain, and gate electrodes on a flexible Kapton substrate using photolithography or screen printing [45].
- Channel Formation: Deposit a film of PEDOT:PSS (e.g., by spin-coating or drop-casting) to form the conductive channel between the source and drain electrodes. Anneal as required.
- Gate Functionalization:
- Option A (Chitosan Matrix): Mix Lactate Oxidase (LOx) with a chitosan solution. Deposit this mixture onto the gate electrode and allow it to crosslink [45].
- Option B (Glutaraldehyde Matrix): Immobilize LOx on the gate using a glutaraldehyde vapor crosslinking method for robust enzyme attachment [45].
- Electrical Characterization:
- Place a droplet of phosphate buffer (e.g., 40 µL) on the device to cover the gate and channel.
- Ground the source and apply a negative drain voltage ((V{DS})). Sweep the gate voltage ((VG)) from 0 V to +1 V to obtain the transistor's transfer characteristics ((ID) vs. (VG)) [45].
- Lactate Sensing Measurement:
- With (V{DS}) and (VG) held at constant values, monitor the drain current ((ID)) over time.
- Sequentially add small volumes (e.g., 5 µL) of lactate solution with increasing concentration to the buffer droplet.
- The enzymatic reaction produces Hâ‚‚Oâ‚‚, which is catalytically reduced at the gate, increasing the effective gate potential and causing a measurable decrease in (ID) [45].
- Data Analysis: Plot the normalized change in (I_D) against the lactate concentration to generate a calibration curve from which sensitivity and limit of detection can be derived.
Protocol: Functionalization of an OECT for Dopamine Sensing
This protocol describes the functionalization of an OECT for ultra-sensitive dopamine detection using aptamer receptors.
Procedure
- OECT Preparation: Start with a fabricated OECT with a PEDOT:PSS channel and a gold gate electrode.
- Aptamer Immobilization: Thiol-modified DNA or RNA aptamers, selected for high specificity and affinity to dopamine, are immobilized onto the gold gate surface via gold-thiol self-assembled monolayer chemistry [44].
- Blocking: Treat the gate surface with a passivating agent (e.g., 6-mercapto-1-hexanol) to block non-specific binding sites.
- Sensing Mechanism: Upon introduction of a sample containing dopamine, the aptamers bind to the target molecules. This binding event alters the electrostatic potential at the gate-electrolyte interface [44].
- Measurement and Readout: The change in gate potential modulates the current in the PEDOT:PSS channel ((I_D)). This amplified signal allows for the quantification of dopamine concentration, even at femto- to pico-molar levels, which aligns with the clinical range found in human body fluids (0.1–10 nM) [44].
Discussion and Application Notes
The integration of OECTs into biosensing platforms offers a powerful tool for biomedical research. The high transconductance of OECTs provides intrinsic signal amplification, enabling the detection of small molecules at physiologically relevant concentrations without the need for additional external amplifiers [5]. The selection of the functionalization strategy—gate, channel, or electrolyte—is critical and depends on the specific analyte and the desired sensor characteristics. Gate functionalization with enzymes or aptamers is a widely adopted and effective method for small molecule detection [5] [44].
A key advantage of OECTs is their compatibility with flexible substrates and low-voltage operation, making them prime candidates for developing wearable and implantable sensors for continuous health monitoring [45] [13]. For instance, OECT-based glucose sensors integrated with near-field communication (NFC) technology have been demonstrated for non-invasive sweat glucose monitoring [43]. Similarly, the development of wearable lactate sensor systems underscores the potential for real-time athletic performance and metabolic health tracking [45].
Future perspectives in this field point towards the creation of multi-analyte sensing platforms by patterning different recognition elements on a single OECT array. Further enhancements in sensitivity, stability, and detection range are anticipated through the development of novel OMIEC materials beyond PEDOT:PSS and the integration of artificial intelligence for advanced data analysis [5] [46] [13]. The convergence of OECT biosensors with implantable drug delivery systems also presents a promising pathway for closed-loop therapeutic platforms, enabling real-time monitoring and automated drug administration for personalized medicine [24].
Organic Electrochemical Transistors (OECTs) have emerged as a revolutionary platform in the field of biosensing, particularly for the detection of macromolecules such as DNA, RNA, and proteins. These biomarkers are crucial for the diagnosis and monitoring of various diseases, including neurodegenerative disorders, cancers, and genetic conditions [5] [47]. OECTs offer significant advantages over traditional detection methods like enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR), including higher sensitivity, lower cost, portability, and the potential for real-time monitoring [47]. Their operation hinges on the unique properties of organic mixed ionic-electronic conductors (OMIECs), which enable the efficient transduction of biological binding events into amplified electrical signals [5] [13]. This application note details the underlying mechanisms, experimental protocols, and key reagents for employing OECTs in the detection of these critical macromolecular biomarkers, framed within the broader context of advancing OECT biosensing research.
OECT Fundamentals and Macromolecular Sensing Mechanisms
The core of an OECT is a three-terminal device comprising a gate electrode, a source electrode, and a drain electrode, with a channel made from an OMIEC material (e.g., PEDOT:PSS) that is in direct contact with an electrolyte [5] [13]. The application of a gate voltage ((VG)) modulates the doping level within the OMIEC channel via the injection or extraction of ions from the electrolyte, thereby controlling the drain current ((ID)) [5]. The key performance metric is the transconductance ((gm = \partial ID/\partial V_G)), which represents the signal amplification efficiency of the device [5] [13].
For biosensing, the primary strategy for detecting macromolecules like DNA, RNA, and proteins is gate functionalization [5]. The gate electrode is modified with specific biorecognition elements (e.g., single-stranded DNA probes, antibodies, or aptamers). When the target macromolecule binds to these elements, it alters the electrochemical interface, leading to a shift in the effective gate potential. This change modulates the channel's conductivity, providing a measurable electrical signal that is correlated with the target concentration [5]. This mechanism allows OECTs to convert specific biological binding events into amplified electrical readouts.
The following diagram illustrates the logical workflow and signaling pathway for macromolecule detection using a functionalized OECT.
Performance Metrics for Macromolecule Detection
The table below summarizes key performance metrics achievable with OECT-based biosensors for the detection of various macromolecules, as reported in recent literature. The high sensitivity and low limits of detection (LOD) highlight the capability of OECTs for advanced diagnostic applications.
Table 1: Performance Metrics of OECT-based Biosensors for Macromolecule Detection
| Target Biomarker | Biorecognition Element | Limit of Detection (LOD) | Linear Detection Range | Key Material/Strategy |
|---|---|---|---|---|
| DNA [5] | Complementary DNA probe | Not Specified | Not Specified | Gate functionalization |
| Proteins (e.g., BRCA-1) [48] | Antibody | 0.04 ng/mL | 0.05 - 20 ng/mL | AuNPs/MoSâ‚‚ nanocomposite |
| Immunological Molecules [13] | Antibody/Aptamer | Not Specified | Not Specified | Functional OECTs |
| General Proteins [5] | Antibody | Not Specified | Not Specified | Gate/channel modification |
Experimental Protocol for OECT-Based Protein Detection
This protocol provides a detailed methodology for fabricating an OECT biosensor and applying it to the detection of a protein biomarker, such as BRCA-1 [48].
Sensor Fabrication and Gate Functionalization
Device Fabrication:
- Substrate Preparation: Clean a glass or flexible substrate (e.g., PET) using oxygen plasma.
- Electrode Patterning: Use photolithography or shadow masking to deposit and pattern source and drain electrodes (typically 100 nm Au with a 10 nm Cr or Ti adhesion layer) [23]. Define the channel dimensions (e.g., Width (W) = 1000 µm, Length (L) = 10 µm).
- Channel Deposition: Spin-coat or drop-cast the OMIEC material (e.g., PEDOT:PSS, often cross-linked with GOPS for stability) onto the channel area [23]. Anneal at 120°C for 1 hour to solidify.
- Gate Electrode: Use a Ag/AgCl reference electrode as the gate or a patterned metal gate.
Gate Functionalization with Nanocomposite:
- Prepare a nanocomposite solution containing molybdenum disulfide (MoSâ‚‚), gold nanoparticles (AuNPs), and chitosan (CS) [48].
- Drop-cast this nanocomposite onto the gate electrode and allow it to dry. The chitosan provides a biocompatible matrix, while the AuNPs and MoSâ‚‚ enhance electron transfer and provide sites for antibody immobilization.
- Immobilize the specific capture antibody (e.g., anti-BRCA-1) onto the modified gate surface via physical adsorption or covalent bonding (e.g., using EDC-NHS chemistry).
- Block non-specific binding sites by incubating the gate with a solution of Bovine Serum Albumin (BSA, 1% w/v) or casein for 1 hour.
- Rinse the functionalized gate gently with phosphate-buffered saline (PBS, pH 7.4) to remove unbound reagents.
Electrical Measurement and Biosensing
Setup: Place the functionalized OECT in an electrochemical cell containing a suitable electrolyte (e.g., 1X PBS, pH 7.4). Connect the source, drain, and gate to a semiconductor parameter analyzer or a custom-built potentiostat [23].
Baseline Measurement:
- Apply a constant drain voltage ((V_{DS}), e.g., -0.5 V) [23].
- Sweep the gate voltage ((VG)) to obtain the transfer characteristics ((ID) vs. (VG)) and output characteristics ((ID) vs. (V{DS})) of the device in the pure buffer solution. Record the baseline drain current ((I{D0})) at your chosen operating point (e.g., (VG) = -0.3 V, (V{DS}) = -0.5 V).
Target Protein Detection:
- Introduce the sample solution containing the target protein (e.g., BRCA-1) to the electrolyte.
- Allow the solution to incubate for a specific time (e.g., 10-30 minutes) to facilitate antigen-antibody binding at the gate.
- Under the same constant (V{DS}) and (VG) as the baseline measurement, monitor the drain current ((I_D)) in real-time.
- The binding event will cause a measurable change in the drain current ((\Delta I_D)).
Calibration and Quantification:
- Repeat the measurement with standard solutions of known target protein concentrations.
- Plot the normalized current response ((\Delta ID / I{D0})) or the absolute (\Delta I_D) against the logarithm of the protein concentration.
- Fit the data to a suitable model (e.g., logistic function) to create a calibration curve for quantifying unknown samples.
The following diagram visualizes this experimental workflow from sensor preparation to signal measurement.
The Scientist's Toolkit: Research Reagent Solutions
Successful implementation of OECT-based biosensors relies on a set of key materials and reagents. The table below lists essential components and their functions in the biosensing process.
Table 2: Essential Research Reagents for OECT-Based Macromolecule Detection
| Reagent/Material | Function/Explanation | Examples / Notes |
|---|---|---|
| OMIEC Channel Material | Serves as the active channel; transduces ionic flux into electronic current. | PEDOT:PSS (most common) [13] [16], PTHS, BBL [13]. |
| Biorecognition Element | Provides specificity by binding the target macromolecule. | Antibodies (for proteins) [48], DNA/RNA probes (for nucleic acids) [5], Aptamers. |
| Nanocomposite Materials | Enhance sensitivity and signal amplification; provide high surface area for bioreceptor immobilization. | Gold Nanoparticles (AuNPs) [48], Molybdenum Disulfide (MoSâ‚‚) [48], Graphene-QDs [48]. |
| Cross-linker | Stabilizes the OMIEC channel, particularly in aqueous environments. | (3-glycidyloxypropyl)trimethoxysilane (GOPS) [23]. |
| Blocking Agent | Reduces non-specific binding, thereby improving signal-to-noise ratio. | Bovine Serum Albumin (BSA), casein, or other proprietary blocking buffers. |
| Electrolyte | Medium for ion transport; forms the ionic circuit of the OECT. | Phosphate Buffered Saline (PBS) [23], or customized biological buffers. |
| Solid-State Gel Electrolyte | Replaces liquid electrolyte for wearable/implantable devices; prevents leakage. | Hydrogels (e.g., PVA, PEG) [16], Ionic Liquid Gels (e.g., [Câ‚‚MIM][EtSOâ‚„]) [16]. |
| Eprociclovir sodium | Eprociclovir sodium, CAS:219657-36-0, MF:C11H14N5NaO3, MW:287.25 g/mol | Chemical Reagent |
| Daphnilongeridine | Daphnilongeridine, MF:C32H51NO4, MW:513.8 g/mol | Chemical Reagent |
Application Notes
Organic Electrochemical Transistors (OECTs) are revolutionizing wearable bioelectronics by merging high-sensitivity biosensing with local signal processing directly at the point of measurement. This in-sensor computing paradigm is critical for developing low-power, continuous health monitoring systems for applications in remote patient monitoring, athletic performance, and personalized medicine [4] [12] [49].
The table below summarizes the key performance characteristics of recent advanced OECT platforms:
Table 1: Performance Comparison of Emerging OECT Platforms
| Platform Characteristic | fPCB-based Flexible OECT [4] | Fiber-based OECT (F-OECT) [12] | OECT-Amplified Biofuel Cell [49] |
|---|---|---|---|
| Key Innovation | Rapid, low-cost fabrication on flexible PCB; integrated in-sensor computing | Fibre geometry for seamless textile integration; high flexibility | Electronic coupling with fuel cells for dramatic signal amplification |
| Transconductance (Gm) | High (enabled by fPCB design) | High (due to high W/L ratio from fibre geometry) | Not Specified |
| Signal Amplification | Native OECT amplification | Native OECT amplification | 1,000 to 7,000-fold signal amplification |
| Stability/Flexibility | Excellent stability & negligible performance change under bending | Stable performance under strain and complex deformations | Stable operation in power-matched mode |
| Primary Applications | Neuromorphic computing, tactile sensing, wearable bioelectronics | Textile-integrated biosensors, wearable & implantable devices | Ultrasensitive detection of biomolecules (e.g., lactate, arsenite in water) |
Core Applications and Principles
Fabrication and Flexibility: A primary advancement is the development of fabrication techniques that lower the barrier to creating robust, flexible OECTs. The use of flexible Printed Circuit Board (fPCB) technology, combined with inkjet printing of the channel and gel electrolyte, allows for the production of devices at a cost of approximately USD 10 per square meter and a fast turnaround time of under 24 hours. This method achieves a device yield close to 100% and maintains performance under bending curvatures, which is essential for comfortable, long-term wearables [4]. Alternatively, Fiber-Based OECTs (F-OECTs) use a fibrillary structure that provides a high surface area and inherent flexibility, enabling their weaving directly into textiles [12].
Signal Amplification for Sensing: A breakthrough in sensitivity has been achieved by electronically coupling OECTs with enzymatic or microbial fuel cells. This configuration separates the bio-recognition event (in the fuel cell) from the signal amplification (in the OECT), allowing each to operate in an optimal environment. This method amplifies weak electrical signals by three orders of magnitude (1,000 to 7,000 times) and improves the signal-to-noise ratio, paving the way for detecting low-concentration biomarkers in complex media [49].
In-Sensor Computing: OECTs are inherently non-linear devices due to ion-diffusion dynamics, making them ideal hardware for mimicking neural behavior. The integration of OECT arrays with signal processing circuits (including a Bluetooth-Low-Energy microcontroller) on a single flexible platform enables real-time biosignal acquisition, processing, and transmission. This allows for local, intelligent data analysis, reducing the need for constant data streaming and lowering overall system power consumption [4].
Experimental Protocols
Protocol: Fabrication of Flexible OECTs via fPCB and Inkjet Printing
This protocol details the rapid prototyping of all-solid-state, flexible OECTs for in-sensor computing applications [4].
Objective: To fabricate a stable, high-yield flexible OECT array suitable for biosensing and neuromorphic computing on skin-conformable substrates.
Materials and Reagents: Table 2: Research Reagent Solutions for fPCB-OECT Fabrication
Item Function/Description Flexible PCB (fPCB) Serves as the device substrate with pre-patterned copper interconnects, source, drain, and gate electrodes. Polyimide (PI) Used as the flexible substrate and encapsulation layer to insulate and protect the copper electrodes. Gold Plating Solution Provides a thin (e.g., 20 nm) protective layer over copper electrodes to enhance electrochemical stability. PEDOT:PSS Ink The organic semiconductor material forming the transistor channel; often modified with crosslinkers for adhesion. Gel Electrolyte (e.g., PEA) Solid-state ion reservoir that gates the transistor; prevents leakage and reaction with underlying copper electrodes. Customizable Inkjet Printer For precise, non-contact patterning of the PEDOT:PSS channel and gel electrolyte onto the fPCB substrate. Step-by-Step Procedure:
- fPCB Electrode Fabrication: Photolithographically pattern copper source, drain, and gate electrodes and interconnects on a polyimide (PI) substrate.
- Electrode Protection and Encapsulation:
- Electroplate a thin layer of gold (~20 nm) over the copper electrodes to mitigate redox reactions.
- Encapsulate the entire electrode array with a second layer of PI, leaving only the contact pads and the active channel/electrolyte regions exposed.
- Channel Patterning: Using a customizable inkjet printer, deposit a precise droplet pattern of PEDOT:PSS ink (formulated with crosslinkers) to form the semiconductor channel between the source and drain electrodes. Cure as required.
- Electrolyte Deposition: Similarly, use inkjet printing to deposit the non-aqueous gel electrolyte (e.g., PEA), ensuring it contacts both the channel and the gate electrode. Crosslink the gel to form a solid-state interface.
- Curing and Final Assembly: Perform a final curing step to ensure adhesion between all functional layers. The device is now ready for characterization and integration into a larger system, such as a flexible patch with a BLE microcontroller.
The following workflow diagram illustrates the key stages of this fabrication process:
Protocol: OECT-Amplified Biofuel Cell for Ultrasensitive Metabolite Detection
This protocol describes the setup for dramatically enhancing the sensitivity of a biofuel cell using an OECT, suitable for detecting metabolites like lactate or environmental contaminants like arsenite [49].
Objective: To detect and amplify the electrical signal from a biofuel cell for highly sensitive, low-power biosensing.
Materials and Reagents:
- Biofuel Cell: Either an enzymatic fuel cell (e.g., with glucose dehydrogenase) or a microbial fuel cell (e.g., with engineered E. coli).
- OECT Components: Gate, source, and drain electrodes; a channel made of a conductive polymer (e.g., PEDOT:PSS).
- Electrolyte: A suitable medium for the OECT (e.g., aqueous solution).
- Potentiostat/Data Acquisition System: To measure electrical output.
Step-by-Step Procedure:
- Prepare the Biofuel Cell: Construct either an enzymatic or microbial fuel cell that generates a current in response to the target analyte (e.g., lactate or arsenite).
- Assemble the OECT: Fabricate a standard OECT on a preferred substrate (e.g., glass slide for miniaturization) with a known channel material.
- System Integration - Cathode-Gate Configuration:
- Connect the cathode of the biofuel cell to the gate electrode of the OECT.
- Connect the anode of the biofuel cell to the source electrode of the OECT.
- This configuration is found to provide the highest signal amplification.
- Operation and Data Collection:
- Expose the biofuel cell to the sample containing the target analyte.
- The resulting current from the fuel cell acts as the gate voltage for the OECT.
- Measure the amplified drain current (IDS) of the OECT, which correlates with the analyte concentration.
- Validation: Calibrate the system using samples with known analyte concentrations. For example, this setup can detect arsenite at concentrations as low as 0.1 µmol/L.
The diagram below illustrates the signal flow and configuration of this amplified sensing system:
Overcoming Practical Hurdles: Stability, Reusability, and Performance Optimization
Organic Electrochemical Transistors (OECTs) have emerged as a leading technology in bioelectronics, particularly for biosensing and neuromorphic computing applications. Their high transconductance, low operating voltage, and inherent biocompatibility make them ideal for interfacing with biological systems. However, a significant challenge that impedes their transition from laboratory research to commercial and clinical applications is ensuring long-term operational stability. The performance of OECTs degrades over time due to factors such as electrolyte evaporation, mechanical stress, and electrochemical instability of electrode materials. This application note addresses these challenges by providing a detailed examination of two cornerstone strategies: the implementation of gel-based electrolytes and advanced electrode protection methods. Framed within the context of a broader thesis on OECT biosensing research, this document synthesizes the most current research findings and provides standardized protocols to help researchers and drug development professionals achieve reproducible and reliable device performance, which is crucial for long-duration biosensing, continuous health monitoring, and implantable device applications.
Gel-Based Electrolyte Systems for Enhanced Stability
Gel electrolytes, including hydrogels and ionogels, have superseded liquid electrolytes as the standard for stable OECTs. They function by immobilizing liquid electrolyte components within a solid or quasi-solid polymer network, thereby combining high ionic conductivity with superior mechanical and environmental stability. Their viscoelastic properties improve mechanical compatibility with biological tissues and flexible substrates, which is paramount for wearable and implantable sensors.
Properties and Classifications of Gel Electrolytes
Gel electrolytes for OECTs are broadly classified based on their polymer matrix and solvent phase. Key properties that dictate their performance include ionic conductivity, elastic modulus, swelling ratio, and adhesion.
- Hydrogels: These consist of a hydrophilic polymer network swollen with water. They offer excellent biocompatibility and high ionic conductivity. A prominent example is the double-network PEDOT:PSS/Polyacrylamide (PAM) organohydrogel, which provides a robust structure capable of withstanding repeated mechanical deformation [10].
- Ionogels: These incorporate an ionic liquid within a solid polymer matrix. They are particularly advantageous for applications requiring non-volatility and wide electrochemical stability windows. Poly(Ionic Liquid) (PIL) ionogels have demonstrated exceptional stability, enabling OECTs that maintain performance over thousands of stretching cycles [10].
- Non-Aqueous Gel Electrolytes: For OECTs fabricated on substrates containing water-sensitive materials (e.g., copper in flexible printed circuit boards), non-aqueous gels are essential. Gel electrolytes like PEA (propylene glycol alginate) have been successfully used to prevent the redox reaction of underlying copper electrodes, thereby enabling all-solid-state, flexible devices [4].
Table 1: Comparison of Gel Electrolyte Types for OECTs
| Gel Type | Key Components | Advantages | Limitations | Exemplary Performance Data |
|---|---|---|---|---|
| Hydrogel | PEDOT:PSS/PAM, Water [10] | High ionic conductivity, excellent biocompatibility, self-healing capability | Potential dehydration, limited temperature stability | Stretchability up to 50%, stability over 10,000 cycles at 30% strain [10] |
| Ionogel | Poly(Ionic Liquid) [10] | Non-volatile, wide electrochemical window, high thermal stability | Potentially lower biocompatibility than hydrogels | Transconductance of 86.4 mS, On/off ratio of 1.2 × 10ⵠ[10] |
| Non-Aqueous Organogel | PEA in organic solvent [4] | Protects water-sensitive electrodes (e.g., Cu), good stability | Lower ionic conductivity than aqueous gels | On/off ratio ~1000, stable performance after 60 gate pulses [4] |
Quantitative Data on Stability Performance
The implementation of gel electrolytes directly translates to quantifiable improvements in device longevity and robustness. The following table summarizes key metrics reported in recent literature.
Table 2: Quantitative Stability Metrics Enabled by Gel Electrolytes
| Stability Metric | Gel Electrolyte System | Device Architecture | Performance Outcome | Reference |
|---|---|---|---|---|
| Cyclic Stability | PEA-based gel [4] | fPCB-based flexible OECT | Negligible performance decay after 60 cycles of gate pulses | [4] |
| Stretching Stability | PIL Ionogel [10] | All-gel OECT | Stable operation after 10,000 stretch-release cycles at 30% strain | [10] |
| Electrochemical Stability | Non-aqueous gel [4] | fPCB OECT with Cu/Au electrodes | Negligible redox current in CV scans from -1 V to +1 V | [4] |
| Long-Term Storage | N/A (Device focused) [31] | NHC-functionalized OECT | Functional gate and device after 24 months of room-temperature storage | [31] |
Electrode Protection and Functionalization Strategies
Electrode degradation is a primary failure mode in OECTs. Strategies to mitigate this include using inert materials, applying protective coatings, and employing robust functionalization chemistries to maintain sensing specificity over time.
Material Selection and Protective Coatings
The choice of electrode material and the use of thin-film barriers are critical for preventing corrosion and unwanted Faradaic reactions.
- Gold-Protected Copper Electrodes: For low-cost, flexible PCB-based OECTs, copper electrodes are economically attractive but electrochemically unstable. A proven solution is to electroplate a thin layer (e.g., 20 nm) of gold onto the copper traces. This layer acts as a conformal barrier, and when coupled with a non-aqueous gel electrolyte, it effectively suppresses copper oxidation, enabling stable device operation within a typical OECT voltage window (-1 V to +1 V) [4].
- Adhesion Promoters: Delamination of functional layers under mechanical stress is a common issue. Incorporating crosslinkers like (3-glycidyloxypropyl) trimethoxysilane (GOPS) into the conducting polymer channel (e.g., PEDOT:PSS) and the gel electrolyte significantly improves adhesion to flexible substrates, preventing delamination over time and under bending conditions [4].
Robust Gate Functionalization Chemistry
Gate electrode functionalization with biorecognition elements is common in biosensing. The stability of this functionalization layer directly determines the sensor's shelf life and operational reliability.
- N-Heterocyclic Carbene (NHC) Linkers: While thiol-based self-assembled monolayers (SAMs) are widely used for gold functionalization, they are prone to oxidation and degradation. NHC linkers form a stronger covalent bond with gold (Au-C dissociation energy of 67 kcal/mol vs. ~45 kcal/mol for Au-S), resulting in ultra-stable SAMs with high thermal, hydrolytic, and oxidative stability [31]. OECTs with NHC-functionalized Au gates have demonstrated stable biosensing performance (e.g., detecting biotin-streptavidin binding) after 24 months of storage at room temperature, a level of longevity difficult to achieve with thiol chemistry [31].
Experimental Protocols
This section provides detailed, actionable protocols for implementing the discussed strategies.
Protocol: Fabrication of Stable Flexible OECTs using fPCB and Gel Electrolyte
This protocol outlines a rapid, low-cost method for creating flexible, all-solid-state OECTs with protected electrodes, adapted from [4].
Research Reagent Solutions & Materials
| Item | Function/Description | Exemplary Product/Composition |
|---|---|---|
| Flexible PCB | Provides substrate, electrodes, and interconnects. | Polyimide substrate with photolithographically patterned 35 µm thick Cu traces. |
| Gold Plating Solution | Forms a protective, electrochemically inert layer on Cu. | Commercial non-cyanide Au plating solution. |
| PEDOT:PSS Ink | Forms the semiconducting channel of the OECT. | Clevios PH1000 with 5% EG, 0.1% DBSA, and 1% GOPS crosslinker. |
| Gel Electrolyte | Solid-state ion reservoir for gating; protects Cu. | PEA (Propylene Glycol Alginate) or similar non-aqueous gel. |
| Inkjet Printer | For precise deposition of channel and electrolyte. | Customizable piezoelectric inkjet printer with alignment stage. |
Methodology
Electrode Fabrication (fPCB Process):
- Source, drain, and gate electrodes are fabricated on a polyimide substrate using standard photolithographic patterning of copper.
- Electroplate a thin layer of gold (≈20 nm) over all copper traces to serve as a protective barrier.
- Encapsulate the electrodes with a second layer of polyimide, leaving only the contact pads and the active channel/gate areas exposed.
Channel Patterning (Inkjet Printing):
- Deposit the crosslinker-enhanced PEDOT:PSS ink onto the channel region between the source and drain electrodes using inkjet printing.
- Anneal the device in an oven at 130°C for 20 minutes to cure the polymer and ensure strong adhesion.
Gel Electrolyte Patterning (Inkjet Printing):
- Precisely print the non-aqueous gel electrolyte (e.g., PEA) to cover the gap between the channel and the gate electrode. The typical feature size is 100 µm.
- Allow the gel to solidify under ambient conditions or as per the manufacturer's instructions.
Validation and Characterization:
- Perform cyclic voltammetry (CV) on the gate electrode in a three-electrode setup to confirm the absence of redox peaks associated with copper.
- Characterize the OECT by measuring output, transfer, and transient response curves to verify performance metrics like on/off ratio and transconductance.
Protocol: NHC-Functionalization of a Gold Gate Electrode for Biosensing
This protocol describes a robust method for functionalizing the gold gate of an OECT for long-term stable biosensing applications, based on [31].
Methodology
Gate Electrode Preparation:
- Clean the fabricated Au gate electrode (e.g., aerosol jet-printed) with oxygen plasma or piranha solution to remove organic contaminants. (Caution: Piranha solution is extremely dangerous and must be handled with extreme care.)
- Rinse thoroughly with deionized water and ethanol, then dry under a stream of nitrogen.
NHC Ligand Synthesis and Deposition:
- Synthesize the NHC ligand (e.g., an imidazolium salt) under a nitrogen environment using Schlenk techniques, as described in [31].
- Dissolve the synthesized NHC ligand in anhydrous acetonitrile.
- Immerse the clean Au gate electrode in the NHC solution for several hours to allow the formation of a self-assembled monolayer via the strong Au-C bond.
Bioreceptor Immobilization:
- After NHC-SAM formation, activate the terminal functional groups of the NHC layer for bioconjugation. This often involves "click chemistry" reactions (e.g., Copper(I)-catalyzed Azide-Alkyne Cycloaddition).
- Incubate the activated surface with a solution containing the biorecognition element (e.g., biotin, an antibody, or a DNA probe) to covalently attach it to the gate surface.
- Rinse the functionalized gate extensively with a suitable buffer to remove any physisorbed molecules.
Stability Validation:
- The functionalized gate can be stored in a dry, dark environment at room temperature.
- Long-term stability can be validated by periodically testing the biosensor's response to a target analyte (e.g., measuring the threshold voltage shift for streptavidin on a biotin-functionalized gate) over months to years.
Visualization of Strategies and Workflows
The following diagrams illustrate the core concepts and experimental workflows described in this document.
Stability Strategy Logic Model
fPCB OECT Fabrication Workflow
The path to reliable, commercial-grade OECT biosensors is paved with strategies that ensure long-term stability. The integration of gel-based electrolytes—whether hydrogel, ionogel, or non-aqueous gel—addresses critical issues of mechanical integrity and environmental robustness. Concurrently, electrode protection strategies, ranging from simple metal plating to advanced functionalization chemistries like NHCs, prevent electrochemical degradation and maintain bio-recognition capabilities over extended periods. The protocols and data synthesized in this application note provide a foundational toolkit for researchers to build upon. Future directions will likely involve the refinement of "smart" responsive gels, the integration of these stable OECTs with energy storage for self-powered devices, and the systematic validation of these systems in real-world clinical and environmental monitoring settings over multi-year timescales.
Organic Electrochemical Transistors (OECTs) represent a groundbreaking technology in biosensing, known for their high sensitivity, low operating voltage, and excellent biocompatibility [5]. A significant challenge in point-of-care (POC) diagnostics, however, has been the development of biosensors that are both highly sensitive and robustly reusable. Traditional regeneration methods, which often rely on pH regulation, heating, or voltage induction to dissociate probes from analytes, frequently lead to repeated surface contamination and rapid sensitivity loss during cyclic use [50]. Physical or chemical etching alternatives can avoid residue but risk damaging the active layer, ultimately limiting device longevity [50].
The Refreshing-in-Sensing (RIS) concept introduces a paradigm shift by enabling synchronous regeneration during the analyte detection process itself [50]. This innovative mechanism, implemented through drug-mediated OECTs (DM-OECTs), allows a single device to achieve multiple sensitive detections of biomarkers, dramatically reducing usage costs and advancing the potential for long-term monitoring applications [51] [50]. By leveraging the unique properties of drug molecules as sensing probes, the RIS process overcomes the traditional trade-off between high sensitivity and excellent reusability, offering unprecedented regeneration cycles exceeding 200 uses while maintaining ultra-high sensitivity [51] [52].
Principles of Drug-Mediated OECTs and the RIS Mechanism
Fundamental OECT Operation
An Organic Electrochemical Transistor (OECT) is a three-terminal device consisting of source, drain, and gate electrodes, with a channel typically made from a conductive polymer like PEDOT:PSS [5]. The device operates through the modulation of channel conductivity via electrochemical doping and dedoping. When a gate voltage ((VG)) is applied, ions from the electrolyte migrate into the channel, altering its doping level and consequently changing the drain current ((ID)) [12] [5]. This mechanism provides OECTs with high transconductance ((g_m)), enabling significant signal amplification even at low operating voltages (<1 V), making them exceptionally suitable for biosensing applications [9] [5].
Drug Molecules as Sensing Probes
The innovative approach of DM-OECTs utilizes drug molecules as sensing probes, integrating therapeutic and diagnostic functions into a single platform. Drug molecules like gefitinib offer several advantages as probes:
- Designable Targeting Ability: Drug molecules can be engineered for specific recognition of various biomarkers [51].
- Stable Molecular Structure: The stable and diverse structure of drug probes provides greater flexibility for refreshing the transducer surface [51].
- Direct Electrical Modulation: Drug probes can directly modulate the transistor's electrical performance through charge transfer with organic semiconductors [51] [50].
In the specific case of gefitinib-functionalized OECTs, the device leverages three key interactions: (1) charge transfer between gefitinib and organic semiconductors, (2) specific targeting between gefitinib and the Epidermal Growth Factor Receptor (EGFR), and (3) conformational flipping of the EGFR-gefitinib complex [50].
The Refresh-in-Sensing Mechanism
The RIS mechanism represents the core innovation that enables exceptional reusability. Unlike conventional approaches that require separate regeneration steps, the RIS process achieves automatic surface refreshment during target analyte detection [51]. The mechanism operates through competitive interactions: when the target analyte (e.g., EGFR) is introduced, it competes with the transducer surface (PEDOT:PSS) for binding with the drug probes (gefitinib) [51]. This competition, combined with the conformational changes in the target protein upon binding, naturally displaces the probes from the sensor surface during the sensing event. After analyte rinsing, the sensor surface is regenerated and can be reloaded with fresh drug probes by simply soaking the device in a gefitinib solution [51].
Experimental Protocols and Methodologies
DM-OECT Fabrication Protocol
Objective: To fabricate a drug-mediated organic electrochemical transistor for reusable biosensing applications.
Materials Required:
- Substrate: Glass or flexible biocompatible polymer
- Channel Material: PEDOT:PSS solution
- Electrodes: Gold (Au) or platinum (Pt) for source and drain; Ag/AgCl for gate electrode
- Drug Probe: Gefitinib solution (1-10 mM in appropriate solvent)
- Biomarker Solution: Recombinant EGFR protein in buffer
- Clinical Samples: Blood serum from non-small cell lung cancer patients (for validation)
Procedure:
- Substrate Preparation: Clean substrate thoroughly with oxygen plasma treatment to ensure surface hydrophilicity.
- Channel Patterning: Spin-coat PEDOT:PSS onto the substrate at 3000 rpm for 60 seconds, followed by annealing at 140°C for 15 minutes.
- Electrode Deposition: Pattern source and drain electrodes (50 nm Au with 5 nm Ti adhesion layer) using photolithography or shadow masking.
- Device Integration: Define channel area (typically 100-500 μm width, 10-50 μm length) between source and drain electrodes.
- Gate Functionalization: Fabricate Ag/AgCl gate electrode with sufficiently large surface area to ensure high gate capacitance.
- Drug Probe Immobilization: Incubate the device in gefitinib solution (5 mM in ethanol) for 2 hours at room temperature, allowing electrostatic adsorption of protonated gefitinib onto PEDOT:PSS surface.
- Rinsing and Storage: Rinse gently with deionized water to remove unbound gefitinib and store in PBS buffer at 4°C until use.
Biosensing and Regeneration Protocol
Objective: To detect EGFR biomarkers and achieve device regeneration through the RIS mechanism.
Procedure:
- Baseline Establishment: Immerse the functionalized DM-OECT in phosphate buffered saline (PBS, pH 7.4) and measure transfer characteristics ((ID) vs. (VG)) at constant drain voltage ((V_D = -0.1) to (-0.5) V) to establish baseline current.
- Sample Introduction: Introduce EGFR sample (5 μL to 50 μL, depending on chamber size) to the electrolyte while continuously monitoring (I_D).
- Real-time Monitoring: Record temporal response of (ID) at fixed (VG) and (VD) (e.g., (VG = 0.2) V, (V_D = -0.3) V) for 5-10 minutes.
- RIS Activation: Rinse the device with PBS buffer (pH 7.4) to remove bound EGFR; the competitive interaction between gefitinib-PEDOT:PSS and gefitinib-EGFR facilitates automatic probe displacement during this step.
- Signal Measurement: Quantify EGFR concentration based on normalized current change ((\Delta I/I_0)) before and after sample introduction.
- Device Regeneration: Soak the device in gefitinib solution (1 mM, 10 minutes) to reload drug probes onto the refreshed surface.
- Cycle Repetition: Repeat steps 2-6 for subsequent sensing measurements.
Critical Parameters:
- Electrolyte composition and pH must be optimized for specific drug probe-biomarker pairs
- Gate voltage should be optimized to maximize transconductance while avoiding irreversible oxidation/reduction
- Drug probe concentration and immobilization time require optimization for each application
Performance Metrics and Quantitative Data
The performance of DM-OECTs implementing the RIS concept demonstrates remarkable improvements in both sensitivity and reusability compared to conventional biosensors. The table below summarizes key performance metrics reported in recent studies.
Table 1: Performance Metrics of RIS-based DM-OECT for EGFR Detection
| Parameter | Value | Context & Significance |
|---|---|---|
| Detection Limit | 5.74 fg mLâ»Â¹ | Ultra-high sensitivity for early cancer diagnosis [52] |
| Regeneration Cycles | >200 uses | Unprecedented reusability reduces cost per test [51] [52] |
| Detection Range | 5.74 fg mLâ»Â¹ to ~ng mLâ»Â¹ | Broad dynamic range for clinical applicability [52] |
| Signal Amplification | 1,000-7,000x | Compared to traditional electrochemical sensors [9] |
| Response Time | <5 minutes | Rapid results suitable for point-of-care testing [51] |
| Selectivity | Excellent for EGFR | Minimal interference from other blood proteins [51] |
The exceptional performance of RIS-based DM-OECTs is further demonstrated through their application in clinical settings. When configured as an 8 × 12 diagnostic array, these devices showed excellent uniformity and reliability when testing clinical blood samples from non-small cell lung cancer patients [52]. The array format enables high-throughput screening while maintaining the exceptional sensitivity and reusability of individual sensors.
Table 2: Comparison of RIS-enabled OECT with Traditional Biosensing Approaches
| Characteristic | RIS-enabled DM-OECT | Traditional Reusable Biosensors | Significance |
|---|---|---|---|
| Regeneration Mechanism | Refresh-in-Sensing (automatic) | pH, heat, or voltage induction (manual) | Simplified workflow, no additional steps [50] |
| Sensitivity Retention | >95% after 100 cycles | Typically <60% after 10 cycles | Consistent performance over time [51] |
| Surface Contamination | Minimal due to competitive displacement | Significant due to reversible adsorption | Maintains sensor accuracy [50] |
| Active Layer Damage | None reported | Common with physical/chemical etching | Extends device lifetime [50] |
| Cost per Test | Extremely low after initial investment | High due to frequent replacement | Economical for long-term monitoring [51] |
Research Reagent Solutions and Materials
Implementing the RIS concept in OECT biosensing requires specific materials and reagents carefully selected for their complementary functions. The following toolkit outlines essential components:
Table 3: Research Reagent Solutions for RIS-OECT Implementation
| Reagent/Material | Function | Application Notes |
|---|---|---|
| PEDOT:PSS | OECT channel material | High conductivity, mixed ion-electron conduction, biocompatible [51] [5] |
| Gefitinib | Drug probe for EGFR detection | Specific targeting, reversible surface binding, charge transfer capability [51] [52] |
| PBS Buffer (pH 7.4) | Electrolyte and rinsing solution | Physiological compatibility, maintains protein stability [51] |
| Recombinant EGFR | Target biomarker for validation | Sensor calibration and performance evaluation [52] |
| Ag/AgCl Ink | Gate electrode fabrication | Non-polarizable gate, stable reference potential [5] |
| Gold/Titanium Evaporation Targets | Source and drain electrodes | High conductivity, electrochemical stability [5] |
Signaling Pathways and Workflow Visualization
The RIS mechanism involves sophisticated interactions between molecular components during the sensing and regeneration process. The following diagram illustrates the signaling pathway and workflow:
Diagram 1: RIS Mechanism Workflow and Molecular Interactions
This workflow visualization illustrates the four key stages of the Refresh-in-Sensing process: (1) initial drug probe immobilization through electrostatic adsorption; (2) sample introduction and competitive target binding; (3) rinsing phase with automatic probe displacement; and (4) probe reloading for subsequent detection cycles. The molecular-level interactions (dashed lines) highlight the charge transfer, specific targeting, and competitive binding that enable the unique refresh-in-sensing capability.
The integration of drug-mediated probes with the Refresh-in-Sensing concept in OECTs represents a transformative approach to reusable biosensing. By addressing the fundamental challenge of maintaining high sensitivity across numerous regeneration cycles, this technology enables cost-effective, long-term biomarker monitoring essential for chronic disease management, cancer therapy monitoring, and personalized medicine.
Future research directions should focus on expanding the library of drug probes for diverse biomarkers, optimizing device architectures for implantable applications, and integrating RIS-OECTs with closed-loop therapeutic systems. The development of standardized fabrication protocols and the exploration of new polymer semiconductors will further enhance device performance and accessibility. As these advancements mature, RIS-enabled OECT biosensors promise to revolutionize point-of-care diagnostics and continuous health monitoring platforms.
Improving Device Homogeneity and Yield on Flexible Substrates
The advancement of organic electrochemical transistor (OECT) based biosensors is fundamentally constrained by the challenge of manufacturing devices on flexible substrates with consistent performance characteristics. Device homogeneity and production yield are critical parameters that determine the reliability, scalability, and eventual commercial viability of flexible OECT technologies for biosensing applications [4] [53]. Variations in electrical performance across devices can lead to inconsistent sensor responses, unreliable data, and compromised diagnostic accuracy—particularly problematic for applications in pharmaceutical development and clinical diagnostics where precision is paramount.
Recent manufacturing innovations have demonstrated promising pathways toward overcoming these challenges. The integration of mature flexible printed circuit board (fPCB) technology with customized inkjet printing has emerged as a particularly viable approach, enabling the production of OECT arrays with demonstrated device yields approaching 100% [4]. Similarly, stencil printing techniques offer alternative fabrication routes that balance cost-effectiveness with performance optimization [54]. This application note synthesizes current protocols and material strategies to provide researchers with standardized methodologies for enhancing manufacturing outcomes in flexible OECT biosensor development.
Fabrication Methodologies for Homogeneous OECT Arrays
fPCB and Inkjet Printing Hybrid Approach
The combination of fPCB technology for electrode fabrication and inkjet printing for functional material deposition represents a robust method for achieving high device homogeneity on flexible substrates [4]. The fPCB process provides photolithographic precision for electrode patterning, while inkjet printing enables controlled deposition of channel and electrolyte materials without compromising the integrity of underlying layers.
Experimental Protocol:
- Substrate Preparation: Begin with a standard 200 μm thick polyimide (PI) substrate cleaned with sequential acetone, isopropyl alcohol, and deionized water rinses, followed by oxygen plasma treatment (100 W, 1 minute) to enhance adhesion [4].
Electrode Fabrication: Pattern source, drain, and gate electrodes via photolithographic patterning of copper conductors (35 μm thickness) on the PI substrate. Electroplate a 20 nm gold protective layer to prevent copper oxidation and improve electrochemical stability [4].
Encapsulation: Apply a second PI encapsulation layer (50 μm thickness) with precisely etched openings to define contact areas for the channel and electrolyte using a programmable laser ablation system [4].
Channel Patterning: Deposit PEDOT:PSS channel material through inkjet printing (e.g., using a Fujifilm Dimatix material printer) with the following optimized parameters:
- Nozzle temperature: 40°C
- Stage temperature: 60°C
- Drop spacing: 25 μm
- Number of passes: 3-5 layers Incorporate crosslinkers (typically 1% v/v GOPS) into the PEDOT:PSS ink formulation to enhance adhesion to the fPCB substrate [4].
Electrolyte Deposition: Pattern a non-aqueous gel electrolyte (e.g., PEA-based) using the same inkjet printing system, ensuring precise alignment over the channel and gate regions. Crosslinkers are similarly added to the electrolyte formulation to prevent delamination during bending operations [4].
Table 1: Key Performance Metrics of fPCB-Fabricated OECTs
| Parameter | Reported Value | Measurement Conditions |
|---|---|---|
| Device Yield | ~100% | Array of 200 devices [4] |
| On/Off Ratio | ~1000 | VGS from -0.2 V to 0.8 V, VDS from -0.1 to -0.6 V [4] |
| Transconductance (gm) | >10 mS | Standard measurement conditions [53] |
| Hole Mobility | 1.1 cm² Vâ»Â¹ sâ»Â¹ | Calculated via Bernards-Malliaras model [4] |
| Feature Size | 100 μm | Channel length/width [4] |
Stencil Printing Optimization Protocol
Stencil printing provides an alternative fabrication approach that is particularly valuable for research environments with limited access to cleanroom facilities [54]. This method enables direct patterning of organic semiconductor channels through precisely fabricated stencils, offering rapid prototyping capabilities with minimal material waste.
Experimental Protocol:
- Stencil Fabrication: Create stencil masks from adhesive vinyl materials using a commercial craft cutter or precision laser cutter. Channel dimensions should be designed with aspect ratios (width:height) not exceeding 5:1 to ensure structural integrity during printing [54].
Ink Formulation: Prepare PEDOT:PSS ink with the following additives:
- 5% (v/v) ethylene glycol (EG) for enhanced conductivity
- 1% (v/v) 4-dodecylbenzenesulfonic acid (DBSA) for improved film formation
- 1% (v/v) (3-glycidyloxypropyl)trimethoxysilane (GOPS) for substrate adhesion [54]
Printing Process: Align the stencil mask on the substrate (flexible PI or PET) and deposit the PEDOT:PSS ink using a precision squeegee at a 45° angle with consistent pressure. Remove the stencil carefully after deposition to avoid edge defects [54].
Annealing Optimization: Implement a thermal annealing process with parameters optimized through experimental design:
- Temperature range: 50-150°C
- Time duration: 5-120 minutes
- Atmosphere: Ambient or nitrogen environment [54]
Table 2: Stencil Printing Experimental Design Parameters and Outcomes
| Factor | Range | Optimal Value | Impact on Performance |
|---|---|---|---|
| Annealing Temperature | 50-150°C | 120°C | Higher temperatures improve film homogeneity but exceed substrate Tg at upper limits [54] |
| Annealing Time | 5-120 minutes | 60 minutes | Sufficient duration for complete solvent evaporation without excessive material degradation [54] |
| Channel Length | 100-500 μm | 200 μm | Balance between current output and switching speed [54] |
| Threshold Voltage | 260 mV | Minimized | Lower operating voltage improves power efficiency and stability [54] |
| On/Off Ratio | 7 × 10³ | Maximized | Essential for clear signal distinction in biosensing [54] |
The Scientist's Toolkit: Essential Materials for OECT Fabrication
Table 3: Key Research Reagent Solutions for Flexible OECT Fabrication
| Material/Reagent | Function | Application Notes |
|---|---|---|
| Polyimide (PI) Substrate | Flexible base material | 200 μm thickness provides optimal balance of flexibility and durability [4] |
| PEDOT:PSS | Organic semiconductor channel material | Enhanced with crosslinkers (GOPS) for improved adhesion [4] [54] |
| Ethylene Glycol (EG) | Conductivity enhancer | 5% v/v in PEDOT:PSS formulation increases charge carrier mobility [54] |
| (3-glycidyloxypropyl)trimethoxysilane (GOPS) | Adhesion promoter | 1% v/v prevents delamination during bending operations [4] [54] |
| Non-aqueous Gel Electrolyte (PEA) | Ion transport medium | Prevents corrosion of copper electrodes; enables stable operation [4] |
| 4-Dodecylbenzenesulfonic acid (DBSA) | Film formation agent | Improves morphology and uniformity of printed channels [54] |
| Caboxine A | Caboxine A, MF:C22H26N2O5, MW:398.5 g/mol | Chemical Reagent |
Characterization and Quality Control Protocols
Electrical Performance Validation
Comprehensive electrical characterization is essential for quantifying device homogeneity across fabricated arrays. Standardized testing protocols ensure consistent evaluation of key performance metrics:
Transfer Characteristics: Sweep gate voltage (VGS) from -0.2 V to 0.8 V while maintaining a constant drain-source voltage (VDS between -0.1 V and -0.6 V). Record drain current (IDS) to generate transfer curves [4].
Output Characteristics: Measure IDS while sweeping VDS from 0 V to -0.6 V at discrete VGS steps (0 V to 0.8 V in 0.1 V increments) [4].
Transient Response: Apply gate voltage pulses (typically 0 V to 0.5 V, 10-60 seconds duration) while monitoring IDS to evaluate switching speed and stability over multiple cycles (≥60 cycles recommended) [4].
Transconductance Calculation: Calculate gm = ∂IDS/∂VGS at constant VDS to assess signal amplification capability—a critical parameter for biosensing applications [4] [53].
Mechanical Stability Assessment
For flexible OECTs intended for wearable or implantable biosensing applications, mechanical robustness under bending stress is crucial:
Bending Test Setup: Mount fabricated devices on 3D-printed testbeds with systematically varied curvature radii [4].
Performance Monitoring: Measure transfer characteristics at each bending radius to quantify performance deviations [4].
Cyclic Fatigue Testing: Subject devices to repeated bending cycles (≥10ⴠcycles recommended) while monitoring key parameters (on/off ratio, transconductance) to assess long-term operational stability [55].
Fabrication Workflow Visualization
Figure 1: Comprehensive workflow for fabricating homogeneous OECTs on flexible substrates, highlighting critical steps where process control directly impacts device yield and performance consistency.
Troubleshooting Common Fabrication Challenges
Even with optimized protocols, researchers may encounter specific issues that compromise device homogeneity and yield:
Poor Adhesion and Delamination: Increase GOPS concentration in PEDOT:PSS formulation (up to 2% v/v) and ensure thorough substrate cleaning and plasma treatment prior to printing [4] [54].
High Device-to-Device Variation: Verify inkjet printer nozzle functionality and implement regular cleaning cycles. For stencil printing, ensure consistent squeegee pressure and angle during deposition [4] [54].
Electrochemical Instability: Confirm complete coverage of copper electrodes with gold protective layer and utilize non-aqueous gel electrolytes to prevent ion-mediated corrosion [4].
Inconsistent Film Morphology: Optimize annealing parameters specifically for your substrate and environmental conditions. Characterize film quality with atomic force microscopy (AFM) to correlate morphology with electrical performance [54].
The protocols and methodologies detailed in this application note provide a comprehensive framework for achieving high device homogeneity and manufacturing yield in flexible OECT biosensors. The integration of fPCB technology with additive printing processes represents a particularly promising pathway, having demonstrated near-perfect yield in array-level fabrication [4]. Similarly, the experimental design approach to stencil printing optimization enables researchers to systematically identify critical process parameters that govern device performance [54].
As OECT technologies continue to advance toward commercial applications in pharmaceutical development and clinical diagnostics, standardized fabrication protocols will play an increasingly critical role in ensuring device reliability and data integrity. The continued refinement of these manufacturing approaches—coupled with rigorous quality control measures—will accelerate the translation of flexible OECT biosensors from research laboratories to real-world applications.
Organic Electrochemical Transistors (OECTs) represent a groundbreaking technology in biosensing and bioelectronics, offering unparalleled advantages due to their biocompatibility, ability to operate in aqueous environments, and high signal amplification capabilities [56] [15]. These devices function through mixed ionic-electronic conduction, where the application of a gate voltage drives ions from an electrolyte into an organic semiconductor channel, electrochemically modulating its doping state and conductivity [56] [15] [16]. The most common channel material is poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS), a conductive polymer known for its stability and relatively high conductivity [56] [54] [16].
Despite their promising characteristics, the widespread adoption and commercialization of OECTs face significant fabrication hurdles. The transition from laboratory-scale devices to robust, commercial-grade products is hampered by challenges related to interlayer adhesion, electrochemical stability, and manufacturing scalability [4] [54]. Adhesion failures and delamination frequently occur between functional layers, particularly on flexible substrates that undergo mechanical stress, or between electrodes and electrolytes, leading to device degradation and failure [4]. Furthermore, conventional microfabrication processes for OECTs are often time-consuming, require expensive cleanroom facilities, and suffer from low device yields, creating a pressing need for rapid prototyping techniques that do not compromise performance [4] [54]. This application note details targeted strategies and standardized protocols to overcome these critical fabrication challenges, enabling the development of reliable, high-performance OECTs for advanced biosensing applications.
Material Solutions and Experimental Protocols
Mitigating Adhesion and Delamination
2.1.1 Chemical Adhesion Promotion The primary strategy for enhancing adhesion between the organic semiconductor channel and the substrate involves the use of chemical crosslinkers. (3-Glycidyloxypropyl)trimethoxysilane (GOPS) is the most prevalent adhesion promoter used in PEDOT:PSS formulations [54]. GOPS functions by forming covalent bonds between the silane group and hydroxylated substrate surfaces (e.g., glass, metal oxides), while its epoxide ring opens to react with the sulfonate groups of PSS, creating a robust, crosslinked network.
Protocol: Incorporating GOPS into PEDOT:PSS Inks
- Solution Preparation: Begin with a pristine PEDOT:PSS aqueous dispersion (e.g., Clevios SV4 or PH1000).
- Additive Mixing: Add secondary dopants to enhance conductivity, typically 3-5% v/v Ethylene Glycol (EG) or DMSO.
- GOPS Addition: Under constant stirring, add GOPS to achieve a final concentration of 1-3% v/v. The solution will appear milky initially.
- Homogenization: Stir the mixture for at least 30 minutes at room temperature until the solution becomes clear and homogeneous.
- Filtration: Filter the final ink through a 0.45 μm syringe filter to remove any aggregates before deposition.
Curing Process: After deposition (e.g., by spin-coating, inkjet, or stencil printing), the film must be thermally cured. A two-stage curing profile is recommended:
- Stage 1: 60-80°C for 5-15 minutes to remove excess water.
- Stage 2: 120-140°C for 30-60 minutes to complete the silanol condensation and epoxide ring-opening reactions, ensuring maximal adhesion and crosslinking [54].
2.1.2 Physical and Architectural Strategies Beyond chemical crosslinking, physical and device architecture strategies are critical for preventing delamination, especially under mechanical stress or in solid-state devices.
Gel Electrolytes: Replacing liquid electrolytes with solid-state gel electrolytes is a highly effective method to mitigate mechanical mismatch and prevent delamination at the electrolyte/channel interface. Gels provide a cohesive, viscoelastic medium that conforms well to other layers and eliminates issues of leakage [4] [16].
- Hydrogels: Networks like poly(vinyl alcohol) (PVA), poly(ethylene glycol) diacrylate (PEGDA), or polyacrylamide (PAAm) swollen with salt solutions (e.g., PBS, NaCl) offer high ionic conductivity and biocompatibility [16].
- Ionic Liquid Gels: Gels incorporating ionic liquids (e.g., [EMIM][TFSI]) provide enhanced thermal stability, non-volatility, and wider electrochemical windows, making them suitable for demanding environments [16].
Substrate Engineering: Using flexible printed circuit board (fPCB) technology with polyimide (PI) substrates creates a robust and mechanically stable platform. The fPCB process allows for the fabrication of electrodes and interconnects that are encapsulated within polyimide layers, providing excellent adhesion and environmental protection [4].
Advanced Rapid Prototyping Techniques
To overcome the limitations of traditional photolithography, several advanced rapid prototyping techniques have been developed, enabling fast, low-cost, and scalable fabrication of OECTs.
2.2.1 fPCB and Inkjet Printing Hybrid Fabrication This hybrid approach leverages the industrial maturity of fPCB for creating electrode arrays and the versatility of inkjet printing for patterning functional materials.
- Protocol: fPCB/Inkjet Prototyping Workflow
- fPCB Fabrication: Design and fabricate source, drain, and gate electrode arrays on a flexible polyimide substrate using standard commercial fPCB processes. A thin gold layer (e.g., 20 nm) is often electroplated on copper traces to enhance electrochemical stability [4].
- Surface Treatment: Clean the fPCB substrate with oxygen plasma (e.g., 100 W for 1 minute) to increase its surface energy and ensure uniform wettability for the printed ink.
- Inkjet Printing of Channel:
- Load the crosslinker-containing PEDOT:PSS ink (as prepared in Section 2.1.1) into a piezoelectric inkjet cartridge.
- Program the printer with the desired channel pattern (typical channel length: 100 μm).
- Print the PEDOT:PSS channel directly onto the fPCB substrate, aligning it between the source and drain electrodes.
- Thermal Curing: Cure the printed device on a hotplate using the two-stage profile described previously.
- Electrolyte Patterning: Deposit the gel electrolyte (e.g., PVA/H3PO4, PEGDA-based) either by inkjet printing or drop-casting, followed by UV or thermal crosslinking as required [4].
This method enables the fabrication of devices with a feature size of 100 μm within 24 hours at a low cost, achieving device yields close to 100% and performance comparable to cleanroom-fabricated devices [4].
2.2.2 Stencil Printing for High-Performance OECTs Stencil printing is a highly cost-effective and fast technique suitable for both rigid and flexible substrates, offering a robust alternative for patterning the OECT channel.
- Protocol: Optimized Stencil Printing
- Stencil and Substrate Preparation: Fabricate a stencil mask from laser-cut adhesive vinyl or metal. Clean the target substrate (e.g., glass, PET, or fPCB).
- Ink Formulation: Prepare a highly viscous PEDOT:PSS formulation with GOPS (1-3% v/v) and EG (5% v/v) to prevent bleeding under the stencil.
- Printing Process: Align and secure the stencil mask on the substrate. Use a squeegee to spread the ink evenly across the stencil, filling the open apertures that define the channel.
- Demasking and Curing: Carefully remove the stencil, leaving the patterned PEDOT:PSS film on the substrate. Cure the film thermally at 120°C for 30-60 minutes [54].
- Performance Optimization: An Experimental Design (e.g., Full Factorial Design) can be employed to optimize key parameters like annealing temperature, annealing time, and channel length to simultaneously achieve a high on/off ratio (>7×10³) and low threshold voltage (~260 mV) [54].
2.2.3 3D Printing and Direct Writing Additive manufacturing techniques offer the highest degree of design freedom for creating complex, three-dimensional OECT architectures.
- Stereolithography (SLA): A composite resin of PEGDA and PEDOT:PSS can be used in SLA printers to fabricate monolithic OECT structures. The process involves digitally designing the 3D object, slicing it into layers, and selectively polymerizing the resin with a UV laser layer-by-layer. Post-printing, devices may require development in a solvent bath and final curing [57].
- Dispense Printing (Direct Ink Writing): This non-contact method is ideal for delicate substrates like nitrocellulose used in lateral flow assays. Using a printer like the Voltera V-One, conductive silver electrodes and PEDOT:PSS channels can be dispensed directly onto the substrate. Printing parameters (pressure, speed, nozzle diameter) are customized for each ink to achieve optimal line definition [58].
Table 1: Comparative Analysis of OECT Rapid Prototyping Techniques
| Fabrication Technique | Key Advantages | Typical Resolution | Reported OECT Performance (On/Off Ratio) | Best Suited For |
|---|---|---|---|---|
| fPCB + Inkjet Printing [4] | Industrial scalability, high yield, integrated circuits, flexibility | ~100 μm | ~1000 | Wearable, integrated biosensing systems |
| Stencil Printing [54] | Very low cost, high speed, simple process, ambient conditions | Tens of micrometers | ~7×10³ | Low-cost, single-use biosensors |
| Inkjet Printing | Non-contact, digital patterning, multi-material printing | ~50 μm | Information Missing | Flexible substrates, customized geometries |
| Stereolithography (SLA) [57] | True 3D geometry, monolithic structures | ~50-100 μm | Information Missing | Custom 3D architectures, implantable devices |
| Dispense Printing [58] | Compatible with porous/delicate substrates, cost-effective printer | ~200 μm | Information Missing | Paper/nitrocellulose-based diagnostic sensors |
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagents and Materials for OECT Fabrication
| Material / Reagent | Function / Role | Example Formulations / Notes |
|---|---|---|
| PEDOT:PSS [4] [54] | Organic semiconductor channel material; conducts electronic charges and allows ion penetration. | Clevios SV4 or PH1000; often modified with secondary dopants and crosslinkers. |
| (3-Glycidyloxypropyl)trimethoxysilane (GOPS) [54] | Crosslinking adhesion promoter; bonds the PEDOT:PSS layer to the substrate. | Typically used at 1-3% v/v in PEDOT:PSS dispersions. Requires thermal curing >120°C. |
| Ethylene Glycol (EG) / DMSO [54] | Secondary dopant; enhances the electrical conductivity of PEDOT:PSS films. | Typically used at 3-5% v/v. Removes excess PSS and improves charge transport. |
| Gel Electrolytes (Hydrogels) [4] [16] | Solid-state ion conductor; replaces liquid electrolytes to prevent leakage and improve mechanical integration. | PVA with LiCl or H3PO4; PEGDA photopolymerizable gel; PAAm; Agarose. |
| Ionic Liquid Gels [16] | Non-volatile, stable solid-state electrolyte; offers wide voltage window and thermal stability. | [EMIM][TFSI] or biocompatible [Ch][Lac] gelled in a polymer matrix (e.g., PNIPAm). |
| Polyimide Substrates [4] | Flexible, thermally stable substrate for fPCB-based OECTs; provides robust mechanical support. | Used in commercial fPCB processes; allows for encapsulated metal traces. |
| Gold-Plated Electrodes [4] | Gate, source, and drain electrodes; provides electrochemical stability versus copper. | Thin layer (e.g., 20 nm) electroplated on fPCB copper traces to prevent oxidation. |
Visualizing Fabrication Workflows and Challenges
The following diagrams illustrate the core fabrication workflows and the key strategies for mitigating adhesion and delamination in OECTs.
Integrated Rapid Prototyping Workflow for OECTs
Integrated Rapid Prototyping Workflow This flowchart outlines the multi-pathway fabrication process for OECTs, highlighting the integration of different rapid prototyping techniques from substrate preparation to a functional device.
Strategies to Mitigate Adhesion & Delamination
Adhesion and Delamination Mitigation Strategies This diagram categorizes the primary solutions for overcoming interfacial failures in OECTs, distinguishing between chemical crosslinking and physical/architectural approaches.
The fabrication challenges of adhesion, delamination, and the need for rapid prototyping in OECT development are significant but surmountable. As detailed in this application note, the strategic implementation of chemical crosslinkers like GOPS, the adoption of solid-state gel electrolytes, and the utilization of advanced rapid prototyping techniques such as hybrid fPCB/inkjet and stencil printing provide a comprehensive toolkit for researchers. These methodologies directly address the key failure modes in OECT fabrication, enabling the creation of robust, high-performance devices suitable for demanding biosensing applications. By standardizing these protocols and understanding the underlying material science, researchers and drug development professionals can accelerate the transition of OECT technology from innovative prototypes to reliable, commercially viable diagnostic and monitoring platforms.
Optimizing Device Geometry and Performance for Specific Bio-environments
Organic Electrochemical Transistors (OECTs) have emerged as a versatile platform for biosensing, offering unique advantages for interfacing with biological environments. Their high transconductance, low operating voltage, and excellent biocompatibility make them particularly suitable for applications ranging from wearable health monitors to implantable diagnostic devices [5] [59]. The performance of OECT-based biosensors is critically dependent on the intricate relationship between device geometry, material properties, and the specific biological environment in which they operate. This application note provides a comprehensive framework for optimizing OECT device geometry and performance for targeted bio-environments, supporting the broader thesis research in OECT biosensing.
Optimizing OECTs requires a multidisciplinary approach that considers how geometric parameters influence device performance metrics and how these parameters must be adapted for different biological contexts. This document presents detailed protocols, quantitative data comparisons, and visualization tools to guide researchers in designing OECTs optimized for specific applications including metabolite sensing, neural recording, and pathogen detection.
OECT Working Principles and Key Performance Metrics
Fundamental Operating Mechanisms
OECTs operate through electrochemical doping processes where ions from an electrolyte penetrate the organic semiconductor channel, modulating its conductivity [15]. A typical OECT consists of three electrodes (gate, source, and drain), a transistor channel comprising an organic mixed ionic-electronic conductor (OMIEC), and an electrolyte that facilitates ion transport [5]. When a gate voltage (VG) is applied, ions are injected into or extracted from the channel material, changing its doping state and consequently the drain current (ID) [5]. This mechanism enables OECTs to efficiently transduce biological signals into measurable electrical outputs.
OECTs can operate in two distinct modes: depletion mode and accumulation mode [15]. Depletion-mode devices (e.g., based on PEDOT:PSS) are intrinsically doped and conductive without gate voltage, with positive gate voltage reducing channel conductivity. Accumulation-mode devices use intrinsically undoped channel materials that become conductive when gate voltage is applied, offering advantages for low-power applications [15].
Critical Performance Parameters
The performance of OECTs for biosensing applications is characterized by several key metrics:
Transconductance (gm): This parameter represents the efficiency of converting small voltage signals at the gate to large current changes in the channel, defined as gm = ∂ID/∂VG [5]. Higher transconductance values enable greater signal amplification and sensitivity.
Response Time: The speed at which the device responds to changes in gate potential, influenced by ion transport dynamics within the channel material [15].
Detection Limit: The lowest concentration of analyte that can be reliably detected, determined by signal-to-noise ratio and device sensitivity [5].
Stability: The ability to maintain consistent performance over time in the target biological environment [13].
The following diagram illustrates the fundamental working principle of an OECT and the key factors influencing its performance in biosensing applications:
Geometric Optimization of OECTs
Fundamental Geometric Relationships
The geometry of an OECT directly influences its key performance parameters, particularly transconductance (gm), which is described by the equation [5] [13]:
[gm = \frac{W d}{L} \mu C^* (V{Th} - V_G)]
Where:
- W = Channel width
- L = Channel length
- d = Channel thickness
- μ = Charge carrier mobility
- C* = Volumetric capacitance
- V({}_{Th}) = Threshold voltage
- V({}_{G}) = Gate voltage
This relationship indicates that geometric parameters (W, L, d) directly influence the transconductance, with wider, thicker channels and shorter lengths generally enhancing gm [5] [13]. However, these geometric changes also affect response times and device footprint, creating optimization trade-offs that must be balanced for specific applications.
Quantitative Geometry-Performance Relationships
Table 1: Influence of Geometric Parameters on OECT Performance Characteristics
| Geometric Parameter | Effect on Transconductance (g~m~) | Effect on Response Time | Impact on Device Integration | Recommended Applications |
|---|---|---|---|---|
| Channel Width (W) | Linear increase with width [5] | Minimal effect | Increased footprint | Metabolite sensing, high-sensitivity detection [13] |
| Channel Length (L) | Inverse relationship (1/L) [5] | Moderate improvement with shorter L | Enables miniaturization | Neural recording, wearable sensors [15] |
| Channel Thickness (d) | Linear increase with thickness [5] [13] | Significant slowing with thicker channels [13] | Minimal footprint impact | Low-frequency sensing, ion detection [37] |
| Gate Electrode Size | Critical for polarizable gates [5] | Affects ion reservoir capacity | Limits miniaturization | Cellular monitoring, electrophysiology [60] |
Bio-Environment Specific Geometric Optimization
Different biological environments present unique constraints and requirements that dictate geometric optimization strategies:
Wearable Sweat Sensors: For sweat-based metabolite monitoring, optimal geometries balance sensitivity with rapid response times. Research demonstrates that OECTs with W/L ratios of 5-10 and channel thicknesses of 200-500 nm provide sufficient sensitivity for glucose and lactate detection while maintaining sub-second response times [13] [61].
Neural Interfaces: Brain-computer interfaces prioritize miniaturization to minimize tissue damage while maintaining signal quality. Devices with channel lengths below 10 μm and thicknesses under 100 nm have demonstrated excellent performance for recording neural activity while enabling dense integration [15].
Implantable Biosensors: For continuous monitoring in tissue, geometric optimization must consider both performance and biocompatibility. Fiber-based OECTs (F-OECTs) with diameters of 10-50 μm enable minimally invasive implantation while maintaining high gm through their unique three-dimensional architecture [15].
Point-of-Care Diagnostics: Rapid pathogen detection (e.g., COVID-19 diagnostics) benefits from geometries that maximize sensitivity for low-abundance targets. Devices with larger gate electrodes and moderate channel thickness (≈1 μm) have achieved detection limits as low as 1 fg/mL for SARS-CoV-2 spike proteins [61].
Performance Optimization for Target Bio-environments
Material Selection and Functionalization Strategies
The selection of channel materials and functionalization approaches must be tailored to the specific target bio-environment and analyte:
Table 2: Material Systems and Functionalization Strategies for Different Bio-environments
| Bio-environment | Channel Material Options | Functionalization Strategy | Key Performance Metrics | Stability Considerations |
|---|---|---|---|---|
| Wearable (Sweat) | PEDOT:PSS, Glycol-modified PEDOT:PSS [13] | Gate modification with glucose oxidase, lactate oxidase [61] | Sensitivity: 0.255 NR/dec (glucose), Detection range: 0.1-10 mM [61] | Mechanical bending stability, humidity resistance [37] |
| Implantable (Tissue) | PEDOT:PSS, BBL, p(g2T-TT), p(g2T-TT)-OH [13] | Biocompatible coatings, enzyme functionalization | Operational stability > 2 weeks, minimal foreign body response [15] | Long-term biofouling resistance, stable performance in vivo [13] |
| Cellular Monitoring | PEDOT:PSS, Pedot-S [13] | Cell-adhesion promoters, extracellular matrix components | Signal-to-noise ratio > 10 dB, response time < 100 ms [60] | Cytocompatibility, non-toxic leaching [60] |
| Point-of-Care (Saliva) | PEDOT:PSS, PTHS [13] [61] | Antibody conjugation (e.g., anti-SARS-CoV-2) [61] | LOD: 1 fg/mL (spike protein), Accuracy: 87.5% (clinical samples) [61] | Shelf stability, minimal non-specific binding [61] |
Signal Amplification and Noise Reduction
Optimizing the signal-to-noise ratio in OECT biosensors requires careful attention to both device design and operational parameters:
Transconductance Maximization: As the primary amplification parameter, gm can be optimized through geometric approaches (previously discussed) and material selection. High μC* products, representing the combination of charge carrier mobility (μ) and volumetric capacitance (C), yield the highest gm values independent of geometry [13]. Recent materials such as p(g2T-TT) have demonstrated exceptional μC products exceeding 500 F/cm·Vâ»Â¹sâ»Â¹ [13].
Gate Electrode Optimization: The choice of gate electrode significantly influences OECT performance. Non-polarizable gates (e.g., Ag/AgCl) enable smaller device footprints without sacrificing performance, while polarizable gates (e.g., Pt, Au) require larger surface areas to maintain sufficient double-layer capacitance [5]. For biosensing applications, gate functionalization with specific receptors enables highly selective detection while leveraging the inherent amplification capability of the OECT.
Operation Voltage Selection: Transfer curve analysis reveals the optimal operating point for maximum sensitivity, typically in the linear region of the transfer curve where gm is maximized [5]. Operating at lower voltages (<0.5 V) minimizes faradaic reactions and improves stability in biological environments while reducing power consumption [59].
Experimental Protocols
Protocol 1: Geometric Optimization for Enhanced Transconductance
Purpose: Systematically optimize OECT geometry to achieve target transconductance values for specific bio-environments.
Materials:
- PEDOT:PSS solution (Clevios PH1000)
- Substrates (glass, PET, or PI)
- Source/Drain electrodes (Au, Cr/Au)
- Gate electrodes (Au, Pt, or Ag/AgCl)
- Electrolyte (PBS, artificial sweat, or artificial cerebrospinal fluid)
Procedure:
- Substrate Preparation: Clean substrates sequentially with acetone, isopropanol, and oxygen plasma treatment (5 min, 100 W).
- Channel Patterning:
- For planar devices: Spin-coat PEDOT:PSS (2000-5000 rpm, 60 s) to achieve desired thickness (100-500 nm).
- Pattern channels using photolithography or shadow masking with varying W (10-500 μm) and L (5-100 μm).
- Anneal at 140°C for 15 min in air.
- Electrode Fabrication:
- Deposit source/drain electrodes (50 nm Au with 5 nm Cr adhesion layer) via thermal evaporation.
- For gate electrodes, fabricate Pt or Au electrodes with areas 10-100× larger than channel area.
- Device Characterization:
- Transfer Characteristics: Sweep VG from -0.6 to 0.6 V with VD constant at -0.6 V.
- Output Characteristics: Sweep VD from 0 to -0.6 V with stepped VG values.
- Calculate gm = ∂ID/∂VG from transfer curves.
- Geometry-Performance Correlation:
- Plot gm versus W/L ratio for devices with constant thickness.
- Plot gm versus d for devices with constant W/L.
- Identify optimal geometry for target transconductance.
Troubleshooting:
- If response times are too slow, reduce channel thickness.
- If gm values are lower than expected, verify channel dimensions and electrode contacts.
- If device-to-device variation exceeds 15%, improve fabrication consistency.
Protocol 2: Bio-functionalization for Specific Targeting
Purpose: Functionalize OECT gates with specific receptors for selective biomarker detection.
Materials:
- Printed OECT devices with Au gate electrodes
- N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)
- Target-specific receptors (enzymes, antibodies, aptamers)
- Ethanolamine blocking solution
- Phosphate buffered saline (PBS), pH 7.4
Procedure:
- Gate Electrode Activation:
- Clean Au gate electrodes with oxygen plasma (2 min, 50 W).
- Immerse in 1:1 v/v EDC/NHS solution (100 mM each) for 30 min.
- Rinse thoroughly with deionized water.
- Receptor Immobilization:
- For enzyme functionalization (e.g., glucose oxidase): Incubate activated gates in 2 mg/mL enzyme solution in PBS for 2 h at 4°C.
- For antibody functionalization: Incubate activated gates in 10 μg/mL antibody solution in PBS for 1 h at room temperature.
- Surface Blocking:
- Treat functionalized gates with 1 M ethanolamine (pH 8.5) for 30 min to deactivate remaining reactive groups.
- Rinse with PBS and store in PBS at 4°C until use.
- Validation:
- Characterize functionalized devices in target analyte solutions.
- Verify specificity using control solutions without target analyte.
- Quantify sensitivity from calibration curves.
Application Notes:
- For COVID-19 detection: Anti-SARS-CoV-2 antibodies enable detection of spike protein with LOD of 1 fg/mL [61].
- For metabolite sensing: Glucose oxidase enables detection range of 100 nM to 50 mM [61].
The following workflow diagram illustrates the complete process for developing optimized OECT biosensors:
Protocol 3: Performance Characterization in Bio-environments
Purpose: Evaluate OECT performance in biologically relevant conditions.
Materials:
- Functionalized OECT devices
- Target biological fluid (artificial sweat, saliva, serum)
- Potentiostat/galvanostat or source measure units
- Data acquisition system
- Environmental chamber (for temperature/humidity control)
Procedure:
- Baseline Characterization:
- Record transfer and output characteristics in PBS.
- Calculate baseline gm, threshold voltage, and ON/OFF ratio.
- Bio-fluid Testing:
- Incubate devices in target bio-fluid at operational temperature (e.g., 32°C for skin, 37°C for internal).
- Record transfer characteristics at t = 0, 1, 6, 24 hours.
- Monitor ID stability over time at fixed VG and VD.
- Sensitivity Assessment:
- Expose devices to calibrated analyte concentrations.
- Record real-time ID response for each concentration step.
- Generate calibration curve (ΔID vs. concentration).
- Calculate sensitivity and limit of detection (LOD = 3σ/slope).
- Selectivity Evaluation:
- Test response to potential interferents at physiologically relevant concentrations.
- Compare response magnitude to target analyte response.
- Stability Assessment:
- Monitor continuous operation for 24-72 hours.
- Calculate signal drift (% change per hour).
- Perform cyclic testing to assess reproducibility.
Data Analysis:
- Calculate key parameters (gm, LOD, sensitivity) for each device.
- Compare performance across different geometries and functionalization.
- Correlate geometric parameters with performance metrics.
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for OECT Biosensor Development
| Reagent/Material | Function/Purpose | Example Specifications | Application Notes |
|---|---|---|---|
| PEDOT:PSS (Clevios PH1000) | Primary channel material | 1.0-1.3% solids in water, conductivity > 800 S/cm | Add 5% DMSO for enhanced conductivity; filter (0.45 μm) before use [13] |
| EGOFET Electrolyte | Gate dielectric/ion source | 0.1 M PBS, pH 7.4 ± 0.1 | Filter sterilize (0.22 μm) for cellular applications [60] |
| Glucose Oxidase (GOx) | Gate functionalization for glucose sensing | ≥100 U/mg, lyophilized powder | Reconstitute in PBS; use EDC/NHS chemistry for immobilization [61] |
| Anti-SARS-CoV-2 Antibody | Gate functionalization for COVID detection | Monoclonal, spike protein specific | Store at 4°C; avoid freeze-thaw cycles; use within 2 months [61] |
| NHS/EDC Crosslinkers | Bioreceptor immobilization | 98% purity, stored desiccated | Prepare fresh solutions; optimize concentration for each receptor [61] |
| Artificial Sweat | Wearable sensor testing | pH 4.5-5.5, lactate, NaCl, urea | Prepare weekly; store at 4°C; warm to 32°C before testing [61] |
| Artificial CSF | Neural interface testing | Ionic composition matching cerebrospinal fluid | Filter sterilize; store at 4°C; use within 48 hours [15] |
Optimizing OECT device geometry and performance for specific bio-environments requires a systematic approach that balances multiple design parameters. The geometric relationships governing transconductance must be carefully balanced against response time requirements and integration constraints for each application. Material selection and functionalization strategies must be tailored to the specific biological interface, whether for wearable, implantable, or point-of-care applications.
The protocols and data presented in this application note provide a foundation for developing OECT biosensors optimized for specific research and clinical applications. As OECT technology continues to advance, further optimization of geometry-performance relationships will enable new capabilities in biosensing, particularly through the development of novel materials with enhanced μC* products and innovative device architectures that maximize sensitivity while minimizing footprint. The integration of these optimized devices with advanced manufacturing approaches such as 3D printing promises to accelerate the translation of OECT biosensors from research laboratories to real-world applications.
Validation and Benchmarking: Assessing OECT Performance Against State-of-the-Art
This application note provides a standardized framework for the performance validation of organic electrochemical transistor (OECT)-based biosensors, specifically addressing the critical parameters of sensitivity, detection limits, and cycle stability. As OECT technology transitions from research laboratories toward industrial-scale applications in bioelectronics and point-of-care diagnostics [62], rigorous and comparable performance assessment becomes paramount. These devices leverage organic mixed ionic-electronic conductors (OMIECs) as channel materials, whose properties directly influence the key performance metrics discussed herein [5] [14]. This protocol is designed for researchers, scientists, and drug development professionals developing OECT biosensors for applications in health monitoring, disease diagnostics, and biological research.
Key Performance Metrics and Typical Values
The table below summarizes the target performance metrics, their definitions, and typical values reported in recent literature, providing a benchmark for validation.
Table 1: Key Performance Metrics for OECT Biosensors
| Performance Metric | Definition and Calculation | Reported Values and Ranges | Influencing Factors |
|---|---|---|---|
| Sensitivity | Change in output signal (e.g., ΔIDS) per unit change in analyte concentration. Derived from the slope of the calibration curve. | Varies by analyte and design. High-sensitivity devices show significant current shift per decade concentration [14]. | Transconductance (gm), gate functionalization, device architecture [5] [30]. |
| Detection Limit (LOD) | The lowest analyte concentration that can be reliably distinguished from background noise. Typically calculated as 3× standard deviation of the blank signal divided by the sensitivity. | Dopamine: ~5 nM [14]EGFR protein: 5.74 fg/mL [30]Glucose: Demonstrated in µM range [63] | Signal-to-noise ratio, non-faradaic interference, binding affinity of receptor [5] [14]. |
| Cycle Stability | The ability of an OECT to maintain its performance over repeated operation cycles. Quantified by the number of cycles before significant performance degradation (e.g., >10% drop in gm or IDS). | >200 cycles for EGFR sensing [30]Stable operation for 30 days in textile OECTs [64] | Operating voltage, OMIEC stability, ion injection/extraction reversibility [64] [65]. |
| Transconductance (gm) | ∂IDS/∂VGS; represents signal amplification efficiency. | Standard PEDOT:PSS: ~90 µS [63]Foam-structured PEDOT:PSS: ~18 mS [63] | Channel material µC* product, geometry (W, L, d), VDS [5] [63]. |
| Response Time | Time required for the device to reach 90% of its final output current after a gate voltage pulse. | Solid PEDOT:PSS film: ~1000 ms [63]Foam-structured PEDOT:PSS: ~300 ms [63] | Ion mobility, channel morphology and thickness [63]. |
Analyte-Specific Detection Performance
The detection performance of an OECT is highly dependent on the target analyte and the functionalization strategy employed.
Table 2: Reported Detection Limits for Various Analytes
| Analyte Class | Specific Analyte | Functionalization Strategy | Reported Detection Limit |
|---|---|---|---|
| Neurotransmitter | Dopamine | Bare Pt or Au gate; Molecularly Imprinted Polymer (MIP) on Pt gate | ~5 nM [14] |
| Protein Biomarker | Epidermal Growth Factor Receptor (EGFR) | PEDOT:PSS channel functionalized with Gefitinib | 5.74 fg/mL [30] |
| Metabolite | Glucose | Gate or channel functionalization with glucose oxidase | Demonstrated in µM range [63] |
| Ions | Na+, Cl-, H+ | Ion-selective membranes | Varies with membrane selectivity [66] |
Experimental Protocols
Protocol 1: Validating Sensitivity and Detection Limit
This protocol outlines the steps for characterizing the sensitivity and lower detection limit of an OECT biosensor.
Principle: The sensitivity is determined from the calibration curve of the device's response to a series of standard analyte solutions. The limit of detection is statistically derived from this curve and the noise level of the measurement system.
Workflow Diagram: Sensitivity and LOD Validation
Materials and Reagents:
- OECT Device: With appropriately functionalized gate or channel [30].
- Analyte Stock Solution: High-purity standard.
- Buffer Solution: Suitable for the analyte and biorecognition element (e.g., PBS, pH 7.4).
- Electrochemical Station: Source meter or potentiostat with multiple channels.
- Data Analysis Software: For curve fitting and statistical analysis.
Procedure:
- Preparation: Prepare a dilution series of the analyte in the appropriate buffer, covering the expected dynamic range (e.g., from 1 nM to 100 µM).
- Baseline Measurement: Immerse the OECT in the pure buffer solution (blank). Apply a constant drain voltage (VDS, typically < 1 V). Sweep the gate voltage (VGS) and record the resulting drain current (IDS) to obtain the baseline transfer characteristic. Alternatively, at a fixed VGS, record IDS over time to establish the baseline current and its noise.
- Sample Measurement: Replace the buffer with the lowest concentration standard solution. Allow the signal to stabilize (typically 1-5 minutes). Record the transfer curve or the steady-state IDS at a fixed VGS.
- Repeat: Rinse the device with buffer and repeat Step 3 for each standard solution in ascending order of concentration.
- Data Analysis:
- For each concentration, extract the metric of interest: either the steady-state IDS at a fixed VGS or the shift in threshold voltage (ΔVT).
- Plot this metric against the logarithm of the analyte concentration.
- Perform a linear regression on the linear portion of the calibration curve. The slope of this line is the sensitivity.
- Calculate the standard deviation (σ) of the response for the blank sample. The LOD is calculated as 3σ/S, where S is the sensitivity obtained from the calibration curve.
Protocol 2: Assessing Cycle Stability and Operational Lifetime
This protocol evaluates the robustness of an OECT biosensor under repeated use, a critical parameter for implantable and continuous monitoring devices.
Principle: The device is subjected to repeated gating cycles that simulate operational conditions. The decay of key performance parameters (e.g., IDS, gm) is monitored over time or cycle number.
Workflow Diagram: Cycle Stability Assessment
Materials and Reagents:
- OECT Device: With channel materials optimized for stability (e.g., treated PEDOT:PSS) [64].
- Electrolyte: Relevant buffer or synthetic biofluid (e.g., artificial sweat).
- Programmable Potentiostat/Source Meter: Capable of applying pulsed waveforms over long durations.
Procedure:
- Initial Characterization: In the chosen electrolyte, measure a full transfer characteristic of the device to determine the initial IDS,ON, IDS,OFF, and peak transconductance (gm, initial).
- Set Up Cycling Parameters: Program the measurement system to apply a continuous pulsed waveform between the gate and source. Key parameters include:
- VGS, high: Sufficient to switch the device to the "ON" or "OFF" state (e.g., 0.5 V for accumulation mode).
- VGS, low: A voltage that returns the device to its baseline state (e.g., 0 V or -0.5 V).
- Pulse Width and Frequency: Define the duration of each state (e.g., 30 s ON, 30 s OFF). Avoid voltages > 0.5 V to maximize lifetime [64].
- VDS: Apply a constant drain voltage.
- Initiate Cycling: Start the pulsed cycling. The total number of cycles or test duration should be defined based on the target application (e.g., 1000 cycles or 30 days).
- Periodic Monitoring: At predefined intervals (e.g., every 100 cycles or every 24 hours), pause the cycling and perform a full transfer characteristic measurement to track the evolution of IDS and gm.
- Data Analysis:
- Plot the normalized IDS,ON and gm as a function of cycle number or time.
- The cycle stability is often reported as the number of cycles after which the performance parameter (e.g., gm) degrades by a certain threshold, typically 10% or 50% of its initial value.
- The operational lifetime is the total duration of stable operation before critical failure.
The Scientist's Toolkit: Key Research Reagent Solutions
The table below lists essential materials and their functions for developing and validating high-performance OECT biosensors.
Table 3: Essential Reagents and Materials for OECT Biosensor Validation
| Category | Item | Function in OECT Biosensing |
|---|---|---|
| Channel Materials | PEDOT:PSS (e.g., Clevios PH1000) | Benchmark OMIEC for depletion-mode OECTs; high stability and mixed conductivity [5] [64]. |
| PEDOT:PSS with additives (Ethylene Glycol, DBSA) | Enhances conductivity and film formation of the channel [64]. | |
| Foam-structured PEDOT:PSS | Increases volumetric capacitance and ion penetration, boosting gm and speeding up response [63]. | |
| Gate Modifiers | Metal Nanoparticles (Au, Pt) | Provide high electrocatalytic activity for redox-based detection of electroactive species [30] [14]. |
| Carbon Nanomaterials (Graphene, CNTs) | High surface area and conductivity for gate modification; enhance sensitivity [30]. | |
| Molecularly Imprinted Polymers (MIPs) | Synthetic receptors on the gate electrode for highly selective recognition of target analytes [14]. | |
| Enzymes (e.g., Glucose Oxidase, Lactate Oxidase) | Biocatalytic recognition layer for specific metabolite detection [5] [67]. | |
| Electrolytes | Hydrogel/Gel Electrolytes (e.g., PVA-based) | Solid-state electrolytes for improved device integration and operational stability; model biological environments [68]. |
| Ion-selective Membranes | Incorporated into electrolyte or on gate to confer ion selectivity [5] [66]. | |
| Stability Enhancers | Sulfuric Acid (for PEDOT:PSS post-treatment) | Improves conductivity and long-term stability of textile OECTs [64]. |
Advanced Configuration: The Potentiometric-OECT (pOECT)
For specific applications, particularly those requiring true potentiometric sensing, the standard OECT configuration has limitations as the gate electrode is not at open circuit potential. The pOECT configuration has been developed to address this [66].
Principle: The pOECT separates the functions of the traditional gate into two electrodes: a sensing gate (GS) held at open circuit potential and a gating gate (GG) that actively applies the doping voltage. This allows the sensing interface to operate under ideal potentiometric conditions, leading to higher accuracy and stability [66].
Schematic Diagram: pOECT Configuration
Validation Consideration: When validating a pOECT, the protocol for sensitivity (Protocol 1) remains similar, but the GS is connected to a high-impedance potentiometer to measure its open circuit potential, while the GG is used to apply VGS. The stability (Protocol 2) is often superior to conventional OECTs for potentiometric sensing applications [66].
Organic Electrochemical Transistors (OECTs) have emerged as a transformative platform in the field of bioelectronics, offering a unique combination of biocompatibility, signal amplification, and operational stability in aqueous environments. This application note provides a detailed comparative analysis of OECTs against two established technologies: traditional electrochemical sensors and Organic Field-Effect Transistors (OFETs). Framed within the context of biosensing research, this document offers structured performance data and detailed experimental protocols to guide researchers and drug development professionals in selecting and implementing the most appropriate sensing technology for their specific applications. The inherent advantages of OECTs, including their high transconductance, low operating voltage, and efficient ion-to-electron transduction, make them particularly suitable for wearable, implantable, and point-of-care diagnostic devices [5] [13].
Fundamental Working Principles and Comparative Analysis
Operational Mechanisms
Organic Electrochemical Transistors (OECTs): OECTs are three-terminal devices featuring a gate electrode, a source electrode, and a drain electrode connected by a channel made of an organic mixed ionic-electronic conductor (OMIEC), typically poly(3,4-ethylenedioxythiophene) doped with polystyrene sulfonate (PEDOT:PSS). The device operates through electrochemical modulation of the channel's conductivity. When a gate voltage (VG) is applied, ions from the electrolyte are injected into the channel bulk, changing its doping level and thereby modulating the drain current (ID) that flows between the source and drain under an applied voltage (VD) [5] [13]. This mechanism provides OECTs with high transconductance (gm), enabling significant signal amplification of small biological signals [5].
Traditional Electrochemical Sensors: Conventional three-electrode systems consist of a working electrode (WE), a counter electrode (CE), and a reference electrode (RE). The analyte interaction occurs at the WE surface, generating a current or potential change that is measured directly. This setup requires a stable RE to maintain a well-defined potential and suffers from challenges in miniaturization, increased electrical noise with smaller electrodes, and limited signal amplification capabilities [66].
Organic Field-Effect Transistors (OFETs): OFETs control the conductivity of a thin organic semiconductor channel via a capacitive field effect from a gate electrode separated by an insulating dielectric. Charge carriers accumulate at the interface between the insulator and semiconductor layers in response to the gate field, modulating current between source and drain electrodes. Unlike OECTs, OFETs primarily involve electronic transport with minimal ionic penetration into the semiconductor bulk [69].
Performance Comparison Table
Table 1: Comparative performance metrics of OECTs, traditional electrochemical sensors, and OFETs for biosensing applications.
| Performance Parameter | OECTs | Traditional Electrochemical Sensors | OFETs |
|---|---|---|---|
| Signal Amplification | High (intrinsic, gm > 10 mS) [5] | None (direct measurement) [66] | Moderate (field-effect modulation) [69] |
| Operating Voltage | Low (< 1 V) [5] | Variable (often higher) | Low to Moderate |
| Detection Limit | Very high (attomole-femtomole demonstrated) [66] | Micromolar to nanomolar | Varies (nanomolar demonstrated) [69] |
| Sensitivity | High (due to high gm and bulk property modulation) [5] [41] | Good (depends on electrode design and chemistry) | Good (interface property modulation) [69] |
| Biocompatibility | Excellent (flexible, compatible with aqueous environments) [5] [12] | Good (but rigid electrodes and RE stability issues) [66] | Good (but typically encapsulated) [69] |
| Miniaturization Potential | High (RE-free operation possible, simple fabrication) [66] | Limited (challenging RE miniaturization) [66] | High (photolithography compatible) [69] |
| Stability in Aqueous Media | Excellent (steady performance in aqueous environments) [69] [5] | Good (but RE fouling and drift) [66] | Moderate (sensitivity to moisture and ions) [69] |
| Response Time | Milliseconds to seconds (depends on channel thickness and ion mobility) [13] | Fast (direct electron transfer) | Fast (electronic process) |
| Flexibility & Wearability | Excellent (compatible with flexible substrates and textiles) [12] | Limited (rigid electrodes and RE systems) | Good (on flexible substrates) [69] |
Mechanism Visualization
Diagram 1: Operational mechanisms of OECTs, traditional electrochemical sensors, and OFETs. OECTs feature direct ion injection into the channel bulk, traditional sensors rely on electron transfer at electrode surfaces, and OFETs operate via field-effect modulation of a semiconductor channel.
OECT Biosensing Mechanisms and Configurations
Primary Sensing Strategies
OECTs employ three principal mechanisms for biomolecule detection, each offering distinct advantages for specific applications:
Gate Functionalization: The gate electrode is modified with biorecognition elements (enzymes, antibodies, aptamers). Binding of target analytes alters the electrochemical potential of the gate, modulating the effective gate voltage (VGeff) and consequently the drain current (ID). This approach provides high sensitivity and has achieved detection limits as low as 10 fM for proteins like SARS-CoV-2 IgG [5] [41].
Channel-Electrolyte Interface Functionalization: The OMIEC channel surface is functionalized to interact with specific analytes. Target binding directly changes the electronic structure or interfacial properties of the channel, altering its conductivity. This method can provide direct label-free detection but may require careful engineering to maintain device stability [5].
Electrolyte Functionalization: The electrolyte itself is modified with enzymes, ion-selective membranes, or suspended cells. Analyte recognition generates ionic species or changes ionic composition that modulate channel doping. This is particularly effective for metabolite sensing (glucose, lactate) and can leverage the OECT's bulk capacitance for signal amplification [5].
Advanced OECT Configurations
Recent advancements have led to specialized OECT configurations that address specific sensing challenges:
Potentiometric-OECT (pOECT): This configuration maintains the sensing electrode under open circuit potential conditions, overcoming the limitation of conventional OECTs where direct voltage application to the gate prevents thermodynamic equilibrium. The pOECT separates the sensing gate (GS) and gating gate (GG), providing higher accuracy, response, and stability compared to conventional OECTs, particularly for potentiometric sensing applications [66].
Fiber-Based OECTs (F-OECTs): These devices leverage fiber structures with high aspect ratios for enhanced mechanical flexibility and seamless textile integration. The fibrillary geometry provides a higher width-to-length (W/L) ratio compared to planar devices, enhancing transconductance and current-driving capability. F-OECTs maintain stable performance under strain, making them ideal for wearable biomedical applications [12].
Dual-Gate OECTs: This configuration employs two functionalized gate electrodes connected in series with OECT channels. Voltage drifts in the two devices cancel each other out, significantly reducing signal drift and improving measurement stability while increasing sensitivity compared to single-gate configurations [41].
Sensing Mechanism Visualization
Diagram 2: OECT biosensing mechanisms and signal transduction pathways. The three primary functionalization strategies convert biological recognition events into measurable electrical signals through different physical mechanisms, all resulting in drain current modulation.
Application-Specific Performance Data
Quantitative Biosensing Performance
Table 2: Performance comparison of OECTs for detection of various biomolecules.
| Target Analyte | Detection Mechanism | Linear Range | Detection Limit | Key Materials |
|---|---|---|---|---|
| Glucose | Enzyme-functionalized gate (Glucose Oxidase) [69] [5] | 0.1 - 8.0 mM [69] | Not specified | PEDOT:PSS channel, Pt/Au gate [69] |
| DNA | Gate functionalization with probe DNA [69] [5] | Not specified | Differentiation of ssDNA/dsDNA via Vth shift [69] | Pentacene semiconductor, SiOâ‚‚ insulator [69] |
| IgG Antibodies | Carboxylic acid-functionalized gate [41] | Not specified | 10 fM (in saliva and serum) [41] | P3HT channel, ITO/PET gate with PT-COOH/PSAA/DDA [41] |
| Ions (Na+, Cl-, H+) | Potentiometric-OECT (pOECT) [66] | Not specified | Higher response vs. 2-electrode setup [66] | PEDOT:PSS channel, ion-selective membranes [66] |
| Lactate | Enzyme-based detection [5] | 0.1 - 8.0 mM [69] | Not specified | PEDOT:PSS channel, lactate oxidase [69] [5] |
| Dopamine | Gate or channel functionalization [5] | Not specified | Not specified | PEDOT:PSS channel, functionalized gate [5] |
Detailed Experimental Protocols
Protocol 1: Fabrication of Standard OECT with PEDOT:PSS Channel
Purpose: To fabricate a standard OECT structure with a PEDOT:PSS channel for general biosensing applications.
Materials:
- Substrate: Glass or flexible polyethylene terephthalate (PET)
- Channel Material: PEDOT:PSS aqueous dispersion
- Electrodes: Gold (Au) or platinum (Pt) for source, drain, and gate
- Electrolyte: Phosphate buffered saline (PBS) or specific biological buffer
- Surface Treatment: Oxygen plasma cleaner or UV-ozone treatment system
Procedure:
- Substrate Preparation: Clean substrate thoroughly with sequential sonication in acetone, isopropanol, and deionized water (15 minutes each). Dry with nitrogen stream.
- Surface Treatment: Treat substrate with oxygen plasma or UV-ozone for 5-10 minutes to enhance hydrophilic properties for better film adhesion.
- Electrode Patterning: Pattern source and drain electrodes (typically 5-100 μm channel length, 100-1000 μm channel width) using photolithography or shadow masking. Thermal evaporation of Au (50 nm) with Ti adhesion layer (5 nm) is recommended.
- Channel Formation: Spin-coat PEDOT:PSS dispersion at 2000-5000 rpm for 30-60 seconds to achieve uniform film (200-500 nm thickness). Alternatively, use drop-casting or spray-coating methods.
- Annealing: Anneal the PEDOT:PSS film at 120-140°C for 15-30 minutes on a hotplate to remove residual water and improve conductivity.
- Device Isolation: Define channel area using photolithography and oxygen plasma etching, or mechanical masking during deposition.
- Gate Electrode Preparation: Fabricate gate electrode using Au, Pt, or Ag/AgCl depending on application requirements.
- Device Encapsulation: Apply epoxy or PDMS encapsulation to define electrolyte well and protect contact areas.
- Characterization: Perform electrical characterization in electrolyte solution using source measure units to obtain transfer (ID vs VG at constant VD) and output (ID vs VD at constant VG) characteristics.
Quality Control: Measure transconductance (gm = ∂ID/∂VG) to ensure values >1 mS for effective signal amplification. Verify device stability by monitoring ID over time at fixed bias conditions with less than 5% variation over 1 hour [5] [13].
Protocol 2: Gate Functionalization for Protein Detection
Purpose: To functionalize OECT gate electrodes with carboxylic acid groups for specific antibody immobilization and antigen detection.
Materials:
- Gate Electrodes: ITO-coated PET substrates or Au gate electrodes
- Functionalization Materials:
- PT-COOH (poly 3-(3-carboxypropyl)thiophene-2,5-diyl)
- PSAA (poly(styrene-co-acrylic acid), random)
- DDA (1,10-decanedicarboxylic acid) for self-assembled layers
- Crosslinkers: EDC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide) and NHS (N-Hydroxysuccinimide)
- Biological Reagents: Target antibodies, blocking agents (BSA or casein)
- Buffers: PBS (pH 7.4), MES (2-(N-morpholino)ethanesulfonic acid) buffer (pH 5.5-6.0)
Procedure:
- Gate Electrode Preparation: Clean ITO/PET substrates by submersion in isopropanol for 15 minutes, dry with nitrogen, and treat with UV-ozone for 30 minutes.
- Functional Layer Application:
- Option A (Polymer Coating): Prepare 1-5 mg/mL solutions of PT-COOH or PSAA in appropriate solvents (DMF for PT-COOH, ethanol/water mixtures for PSAA). Spin-coat at 2000-4000 rpm for 30-60 seconds to form uniform films.
- Option B (Self-Assembled Monolayer): Immerse Au gate electrodes in 1-5 mM DDA solution in ethanol for 12-24 hours to form self-assembled monolayer. Rinse thoroughly with ethanol to remove physisorbed molecules.
- Activation of Carboxylic Groups: Prepare fresh activation solution containing 20 mM EDC and 10 mM NHS in MES buffer (pH 6.0). Incubate functionalized gates in activation solution for 30-60 minutes at room temperature with gentle agitation.
- Antibody Immobilization: Rinse activated gates with PBS (pH 7.4) and immediately incubate with antibody solution (10-100 μg/mL in PBS) for 2 hours at room temperature or overnight at 4°C.
- Blocking: Incubate functionalized gates with 1% BSA or casein solution in PBS for 1 hour to block non-specific binding sites.
- Validation: Characterize functionalization success using contact angle measurements, X-ray photoelectron spectroscopy, or electrochemical impedance spectroscopy.
- OECT Integration: Incorporate functionalized gate into OECT setup and perform sensing experiments in relevant biological fluids (serum, saliva, buffer).
Applications: This protocol enables detection of human IgG, SARS-CoV-2 antibodies, and other protein biomarkers with detection limits reaching 10 fM [41].
Protocol 3: Potentiometric-OECT (pOECT) Configuration for Ion Sensing
Purpose: To implement pOECT configuration that maintains the sensing electrode under open circuit potential conditions for accurate potentiometric measurements.
Materials:
- Standard OECT components (as in Protocol 1)
- Additional Electrodes: Separate sensing gate (GS) and gating gate (GG) electrodes
- Ion-Selective Membranes: Appropriate ionophores for target ions (Na+, K+, Cl-, H+)
- Reference Electrode: Optional Ag/AgCl reference for validation
Procedure:
- Standard OECT Fabrication: Fabricate OECT with PEDOT:PSS channel following Protocol 1.
- Gate System Modification: Replace single gate electrode with two separate electrodes:
- Sensing Gate (GS): Functionalize with ion-selective membrane or specific biorecognition element. This electrode serves as reference for the applied gate voltage.
- Gating Gate (GG): Use inert electrode (Pt or Au) as counter electrode responsible for actively applying doping voltage.
- Electrical Connections:
- Connect drain to WE1 terminal of potentiostat
- Connect source to RE1/CE1 terminal
- Connect sensing gate (GS) to RE2 terminal
- Connect gating gate (GG) to CE2 terminal
- Measurement Protocol:
- Apply fixed VD (typically -0.1 to -0.5 V for p-type OECTs)
- Sweep VG while monitoring ID
- Ensure gate current (IGS) remains minimal (< 1 nA) to maintain near-open circuit conditions
- Calibration: Expose GS to standard solutions with known target ion concentrations to establish calibration curve of ID response vs. concentration.
- Validation: Compare pOECT response with traditional 2-electrode potentiometric measurements to verify enhanced accuracy and stability.
Advantages: This configuration eliminates measurement inaccuracies associated with conventional OECT-based potentiometric sensors, allows implementation of high-impedance sensing electrodes, and provides higher response than 2-electrode setups while maintaining accuracy without a reference electrode [66].
The Researcher's Toolkit: Essential Materials and Reagents
Table 3: Key research reagents and materials for OECT fabrication and functionalization.
| Category | Specific Materials | Function/Purpose | Examples/Notes |
|---|---|---|---|
| Channel Materials | PEDOT:PSS [13] | Primary OMIEC for ion-to-electron transduction | Commercial formulations (Clevios); often require secondary doping with solvents [13] |
| P3HT (poly(3-hexylthiophene-2,5-diyl)) [41] | p-type semiconductor for channel | Used with non-aqueous electrolytes; lower ion permeability than PEDOT:PSS [41] | |
| n-type OMIECs (e.g., BBL, NDI-T2 copolymers) [5] | Enable complementary circuits and enhanced sensing modalities | Emerging materials with improving stability in aqueous environments [5] | |
| Gate Functionalization | PT-COOH [41] | Semiconducting polymer with carboxyl groups for biomolecule immobilization | Allows ion penetration into bulk; altered charge distribution upon binding [41] |
| PSAA (poly(styrene-co-acrylic acid)) [41] | Insulating polymer with carboxyl groups | Biomolecule binding creates interfacial voltage change without bulk effect [41] | |
| DDA (1,10-decanedicarboxylic acid) [41] | Forms self-assembled monolayer for oriented bioreceptor presentation | Creates ultra-thin molecular layers with accessible carboxylic groups [41] | |
| Crosslinking Chemistry | EDC/NHS chemistry [41] | Activates carboxylic groups for amide bond formation with biomolecules | Standard bioconjugation method for antibody/antigen immobilization [41] |
| Electrolytes | Physiological buffers (PBS) [13] | Aqueous ion source for OECT operation | Compatible with biological samples; maintain pH and osmolarity [13] |
| Gel electrolytes (hydrogels) [37] | Solid-state electrolytes for wearable devices | Offer improved mechanical compatibility and stability [37] | |
| Substrates | Flexible polymers (PET, PI) [41] | Support material for flexible OECTs | Enable wearable and implantable form factors [41] |
| Textile fibers [12] | Integration platform for wearable bioelectronics | F-OECTs maintain performance under mechanical deformation [12] |
OECTs represent a significant advancement in biosensing technology, offering distinct advantages over traditional electrochemical sensors and OFETs through their unique combination of high signal amplification, biocompatibility, and operational stability in aqueous environments. The protocols and comparative data presented in this application note provide researchers with practical guidance for implementing OECT-based biosensing platforms across a range of applications from medical diagnostics to environmental monitoring. As material innovations continue to enhance OECT performance and stability, these devices are poised to play an increasingly important role in the development of next-generation wearable, implantable, and point-of-care biosensing systems. Future development directions include leveraging multi-responsive hydrogel electrolytes for intelligent sensor designs, integrating OECTs with energy storage devices for self-powered applications, and advancing wireless communication functionalities for real-time health monitoring [37].
Organic Electrochemical Transistors (OECTs) have emerged as a transformative technology in bioelectronics, particularly for wearable and implantable biosensing applications. Their ability to maintain functionality under mechanical stress is paramount for real-world adoption. This Application Note provides a standardized framework for evaluating the flexibility and endurance of OECT-based biosensors, detailing quantitative performance metrics under bending conditions and providing validated experimental protocols. The content is structured to support research and development activities within the broader context of OECT biosensing, aiming to ensure reliable data acquisition and device performance in dynamic physiological environments.
Quantitative Performance Benchmarks of Flexible OECTs
The performance of flexible OECTs is quantified through key metrics such as transconductance (gm), on/off ratio, charge carrier mobility (μ), and device stability under mechanical deformation. The following table summarizes the performance of various state-of-the-art flexible OECT configurations as reported in recent literature.
Table 1: Performance Metrics of Flexible OECTs under Mechanical Strain
| Device Configuration / Material | Key Performance Metrics (Unstrained) | Strain Tolerance / Bending Conditions | Performance Retention / Endurance | Primary Application Focus |
|---|---|---|---|---|
| fPCB-based, Gel-Gated OECT [4] | gm: ~1 mS (scale-dependent); On/Off: ~1000; μ: 1.1 cm² Vâ»Â¹ sâ»Â¹ | Negligible change in transfer curves & gm under various bending curvatures. | Stable transient response after 60 gate pulse cycles. Device yield close to 100% in 200-device array. | Integrated in-sensor computing systems, wearable bioelectronics. |
| Stretchable All-Gel OECT [10] | gm: 86.4 mS; On/Off: 1.2 × 10âµ; μC*: 7118.6 μF Vâ»Â¹ sâ»Â¹; μ: 5.7 cm² Vâ»Â¹ sâ»Â¹ | Stretchability up to 50% strain. | High stability over 10,000 stretch-release cycles at 30% strain. | Electronic skins, tactile perception for robotic hands, stretchable gas sensors. |
| Stretchable OECT (HKU) [70] | Miniaturized to 100 μm channel size. | Stretchability over 50%. Conformable to human skin. | N/A | In-sensor edge computing for AI-powered wearables, gesture prediction (~90% accuracy). |
| Vertical Corbino OECT (n-PBDF) [71] | gm: 374 mS; μC*: 1787 F cmâ»Â¹ Vâ»Â¹ sâ»Â¹ | Stable performance at a tight bending radius of 5 μm. | Negligible current/gm change after 1000 bending cycles at 5 μm radius. Stable after UV and autoclave sterilization. | Skin-conformal, long-term biosignal monitoring (e.g., ECG). |
Experimental Protocols for Flexibility and Endurance Evaluation
Protocol: Static Bending Test
Objective: To evaluate the effect of constant mechanical deformation on OECT performance. Application: Simulating device operation on fixed-curvature body parts (e.g., limbs).
- Equipment Setup: Prepare 3D-printed or custom-fabricated testbeds with defined radii of curvature (e.g., simulating wrist or elbow bends) [4].
- Device Mounting: Laminate the flexible OECT device onto the testbed, ensuring full conformal contact without introducing external stress.
- Electrical Characterization: Under the static bent state, measure the device's transfer characteristics (ID vs. VG at constant VD) and output characteristics (ID vs. VD at constant VG).
- Data Analysis: Extract key performance parameters (gm, on/off ratio, threshold voltage VT) and compare them with the flat state values. Calculate percentage retention for each parameter.
Protocol: Dynamic Fatigue Test
Objective: To assess the long-term mechanical robustness and operational stability of OECTs under repeated deformation. Application: Validating durability for use in high-mobility applications (e.g., joint monitors, wearable patches).
- Equipment Setup: Utilize a motorized cyclic bending/stretching stage. The strain rate and maximum strain should be programmable and relevant to the target application (e.g., 30% strain for skin-worn devices) [10].
- Initial Characterization: Measure the baseline OECT performance (transfer curve and transient response).
- Cyclic Loading: Subject the device to a predefined number of stretch-release cycles (e.g., 1,000 to 10,000 cycles) [10] [71].
- In-situ/Post-test Monitoring: At regular intervals (e.g., every 500 or 1000 cycles), pause the test and measure the OECT performance parameters.
- Failure Analysis: Document the number of cycles at which performance degrades beyond a specified threshold (e.g., >10% drop in gm or on/off ratio). Inspect for physical damage like cracks or delamination.
Protocol: Conformability and Motion Artifact Assessment
Objective: To validate performance and signal fidelity when deployed on skin or complex surfaces. Application: Ensuring high-quality data acquisition in electrophysiological sensing (ECG, EMG).
- Device Integration: Fabricate ultraflexible OECTs on thin (e.g., a few micrometers) substrates like polyurethane (PU) to ensure softness and skin conformity [71].
- On-Skin Deployment: Attach the device to the target skin location (e.g., chest for ECG, forearm for EMG) using a medical-grade adhesive.
- Signal Acquisition: Record biosignals (e.g., ECG) while the subject is at rest and during movement [70].
- Data Analysis: Calculate the Signal-to-Noise Ratio (SNR) of the recorded signals. For ECG, a benchmark SNR of 38.1 dB over 7 days has been demonstrated with conformable OECTs [71]. Analyze the data for baseline drift or noise spikes characteristic of motion artifacts.
The Scientist's Toolkit: Essential Materials and Reagents
The successful fabrication and operation of high-performance, flexible OECTs rely on a specific set of functional materials.
Table 2: Key Research Reagent Solutions for Flexible OECTs
| Material / Reagent | Function / Role in Flexible OECTs | Examples & Key Characteristics |
|---|---|---|
| Semiconducting Polymer Gel | Forms the active channel of the transistor; governs electronic charge transport and interacts with ions from the electrolyte. | PEDOT:PSS/PAM Organohydrogel: A double-network gel providing high conductivity and stretchability [10]. p(g2T-T) and BBL gels: Enable all-gel OECTs for p-type and n-type operation, respectively [10]. |
| Ionogel Electrolyte | Serves as the ion-conducting medium (gate dielectric); facilitates the electrochemical doping/dedoping of the channel. | Poly(Ionic Liquid) Ionogel: Offers high ionic conductivity, non-volatility, and stability, preventing leakage in solid-state devices [10]. PEA-based Gel: A non-aqueous gel that protects underlying copper electrodes from redox reactions in fPCB-based OECTs [4]. |
| Flexible Substrate | Provides the mechanical foundation for the device; determines baseline flexibility, weight, and conformability. | Polyimide (PI): High thermal stability and mechanical strength, used in fPCB processes [4] [55]. Polyurethane (PU): Offers high stretchability and skin conformability for ultraflexible devices [71]. |
| Stretchable Conductors | Form the source, drain, and gate electrodes; must maintain electrical continuity under strain. | Gold-coated Copper (on fPCB): Gold provides biocompatibility and electrochemical stability, while copper offers high conductivity. The thin, patterned metal on a flexible PI substrate enables bendability [4]. |
| Crosslinkers | Enhance the mechanical robustness and adhesion between functional layers, preventing delamination during bending. | N,N′-methylenebisacrylamide (MBA): Used as a crosslinker in PEDOT:PSS/PAM gels to form a stable 3D network, improving adhesion to substrates [4] [10]. |
Visualizing Experimental Workflows and Material Systems
The following diagrams illustrate the core experimental workflow for endurance testing and the functional interrelationships within a standard all-gel OECT material system.
Diagram: OECT Flexibility and Endurance Testing Workflow
Diagram: All-Gel OECT Material System and Function
The quantitative data and standardized protocols outlined in this document provide a foundation for rigorously evaluating the mechanical and operational endurance of OECTs. The advancement of material systems, particularly all-gel architectures and stretchable composites, is consistently pushing the boundaries of what is possible, enabling devices that are not only highly sensitive but also exceptionally durable and conformable. By adopting these application notes, researchers and developers can accelerate the transition of robust OECT-based biosensors from laboratory prototypes to reliable tools in clinical diagnostics, drug development, and long-term health monitoring.
Organic Electrochemical Transistors (OECTs) have emerged as a transformative biosensing technology due to their high sensitivity, low operating voltage, and excellent biocompatibility [30] [5]. Their ability to amplify small potential changes at the electrolyte interface into significant changes in channel current makes them exceptionally sensitive to biochemical species in complex biological matrices [30]. This application note details the clinical validation protocols and performance metrics for OECT-based biosensors when deployed in patient-derived samples and complex biofluids, providing a framework for researchers and drug development professionals to implement these technologies in diagnostic and monitoring applications.
The volumetric capacitance and mixed ionic-electronic conduction properties of OECTs enable superior signal transduction in biological environments [30]. Their mechanical flexibility and compatibility with aqueous environments further facilitate direct integration with biological systems for wearable and implantable monitoring [72] [73]. This document synthesizes recent advances in OECT clinical validation, with particular emphasis on analytical performance in real-world biological samples.
OECT Biosensing Mechanisms and Clinical Translation
Fundamental Operating Principles
OECTs typically consist of a conducting polymer channel (most commonly PEDOT:PSS), source and drain electrodes, and a gate electrode, all in contact with an electrolyte solution [5]. The fundamental operating mechanism involves electrochemical doping and dedoping of the channel material via ions from the electrolyte, modulated by the gate voltage [72]. When a positive gate voltage is applied in PEDOT:PSS-based OECTs, cations migrate into the channel, compensating for the sulfonate anions of PSS and causing dedoping of the conducting polymer, which decreases drain current [72]. This mechanism provides high transconductance (gm), a key figure of merit representing amplification capability [30] [5].
The sensing mechanism can be described by the Bernards model, which equates OECTs with a combination of an electronic circuit and an ionic circuit [5]. For biosensing applications, three primary functionalization strategies enable target detection:
- Gate functionalization: The gate electrode serves as a recognition site where electron transfer from redox reactions or capacitance variations from binding events modulate the effective gate potential [5].
- Channel-electrolyte interface functionalization: The target analyte reacts with the functionalized channel surface or bulk, altering channel conductivity [5].
- Electrolyte functionalization: Enzymes, ion-selective membranes, or suspended cells integrated into the electrolyte provide specificity [5].
Of these approaches, gate electrode modification is most common as it enhances selectivity and sensitivity without affecting channel performance [30].
Clinical Translation Advantages
OECTs offer several distinct advantages for clinical applications:
- Amplification capability: OECTs provide on-site amplification directly at the bioelectronic interface, improving both limits of detection (LOD) and signal-to-noise ratio compared to conventional electrochemical sensors [30].
- Low operational voltage: Operation typically below 1V ensures safety for biological tissues and compatibility with portable power sources [5].
- Flexibility and miniaturization: Compatible with flexible substrates, OECTs enable conformable interfaces with biological tissues and development of implantable devices [30] [73].
- Multi-analyte detection: Sensor arrays with different functionalizations enable simultaneous monitoring of multiple biomarkers in small sample volumes [72].
The following workflow illustrates the clinical validation pathway for OECT-based biosensors:
Performance Metrics in Complex Biofluids
Ion Detection in Saliva
Saliva has emerged as an attractive non-invasive biofluid for health monitoring, containing various electrolytes and biomarkers at physiologically relevant concentrations [72]. A flexible multi-ion sensing system based on OECTs demonstrated capability for simultaneous detection of sodium (Naâº), potassium (Kâº), and calcium (Ca²âº) ions in human saliva [72]. The system incorporated ion-selective membranes (ISMs) containing specific ionophores deposited onto the OECT structure to provide selectivity.
Table 1: Performance metrics for multi-ion detection in saliva using OECTs [72]
| Analyte | Detection Range (mM) | Sensitivity (mA/decade) | Selectivity Coefficients (-logk) | Clinical Relevance |
|---|---|---|---|---|
| K⺠| 3.4 - 21 | >1.0 | >3.3 (against Naâº) | Dehydration, electrolyte imbalance |
| Na⺠| 0.9 - 13.7 | >1.0 | Not specified | Cardiovascular disorders, metabolic conditions |
| Ca²⺠| 0.69 - 2.45 | >1.0 | >3.4 (against Ca²âº) | Bone metabolism, hormonal regulation |
The system employed machine learning algorithms to mitigate cross-interference among ions in the complex saliva matrix, significantly improving detection accuracy [72]. This approach highlights the importance of advanced data processing for reliable measurements in multi-analyte environments.
Metabolite and Biomarker Detection
OECTs have been successfully applied to detect various metabolites and biomarkers in different biofluids, with performance validated in clinically relevant ranges:
Table 2: OECT performance for metabolite detection in complex biofluids [30] [58] [5]
| Analyte | Biofluid | Detection Range | Limit of Detection (LOD) | Functionalization Strategy |
|---|---|---|---|---|
| Dopamine | Buffer solutions | 0.01 - 1 mM | 0.01 mM | PEDOT:PSS extended gate [58] |
| Glucose | Blood, saliva | 0.05 - 20 mM | Not specified | Enzyme-functionalized gate (glucose oxidase) [5] |
| Lactate | Sweat, ISF | 1 - 30 mM | Not specified | Enzymatic (lactate oxidase) or MIP-based approaches [67] |
| EGFR protein | Blood | 5.74 fg/mL - 100 pg/mL | 5.74 fg/mL | Channel functionalized with anticancer drug gefitinib [30] |
| K⺠ions | Xylem sap (plants) | 10â»âµ - 10â»Â¹ M | 10â»âµ M | Ion-selective membrane with valinomycin [73] |
Notably, the detection of epidermal growth factor receptor (EGFR) in blood samples from healthy donors and patients demonstrated the clinical utility of OECTs for cancer monitoring, with exceptional sensitivity and reusability for over 200 cycles [30].
Long-term Stability and Implantable Applications
For implantable applications, stability in biological environments is crucial. Implantable ion-selective OECTs for potassium monitoring in plant xylem sap have demonstrated operational stability over five weeks, indicating their potential for long-term biomedical monitoring [73]. These devices maintained high sensitivity (215 µA/decade) and excellent selectivity (>1000-fold for K⺠over interfering ions like Naâº, Ca²âº, and Mg²âº) throughout the testing period [73].
The following diagram illustrates the operational mechanism of an ion-selective OECT:
Experimental Protocols
OECT Fabrication on Flexible Substrates
Protocol: Fabrication of flexible OECTs for clinical sampling
Materials: PEN (polyethylene naphthalate) or PI (polyimide) substrate, titanium (10 nm) and gold (100 nm) for electrodes, PEDOT:PSS solution (Clevios PH 1000), ethylene glycol, (3-glycidyloxypropyl) trimethoxysilane cross-linker, ion-selective membrane components [72].
Method:
- Electrode patterning: Deposit Ti/Au electrodes (10 nm/100 nm) on flexible PEN substrate using thermal evaporation. Typical channel dimensions: length = 100 µm, width = 1000 µm [72].
- Encapsulation: Pattern PDMS layer above electrode layer, encapsulating metal wiring while exposing semiconductor and gate regions [72].
- Channel formation: Drop-cast prepared PEDOT:PSS solution (0.025 µL) between source and drain electrodes. Dry on hotplate at 120°C for 30 minutes. Final thickness approximately 600 nm [72].
- Functionalization: For ion-selective OECTs, drop-cast ion-selective membrane cocktail (0.8 µL) onto region above semiconductor channel. Dry at ambient temperature overnight [72].
- Conditioning: Condition channel in 10â»Â² M target ion solution for 2 hours before initial use [72].
Alternative rapid fabrication approach:
For printable OECTs on nitrocellulose membranes:
- Print silver electrodes using dispense printer with disposable nozzle (internal diameter = 230 µm). Bake at 110°C for 15 minutes [58].
- Deposit PEDOT:PSS layers using aligned printing function with 215 µm nozzle. Bake at 110°C for 30 minutes [58].
- Passivate electrodes with 60 nm Al₂O₃ layer via e-beam evaporation [58].
- Apply solid-state electrolyte and cross-link via UV exposure for 20 seconds [58].
- Create hydrophobic barrier around active components using permanent marker to prevent contamination [58].
Gate Functionalization Procedures
Protocol: Enzyme-based functionalization for metabolite detection
Materials: Glucose oxidase (for glucose sensors), lactate oxidase (for lactate sensors), glutaraldehyde, BSA (bovine serum albumin), Nafion solution [5] [67].
Method:
- Prepare enzyme solution: 10 mg/mL enzyme in phosphate buffer (pH 7.4).
- Add cross-linker (0.25% glutaraldehyde) and stabilizer (1% BSA).
- Drop-cast 5-10 µL enzyme mixture onto gate electrode.
- Allow to cross-link for 2 hours at 4°C.
- Apply Nafion membrane (0.5-1% solution) to reduce interferent effects.
- Cure at room temperature for 1 hour [67].
Protocol: Ion-selective membrane preparation
Materials: Poly(vinyl chloride) (PVC), plasticizer (2-nitrophenyl octyl ether), ionophore (e.g., valinomycin for Kâº), tetrahydrofuran [72] [73].
Method:
- Prepare polymeric matrix: 18.7 mg PVC (26.8 wt%) + 49 mg plasticizer (66.7 wt%) [72].
- Add ionophore: 3 mg (6.5 wt%) of appropriate ionophore (potassium ionophore III for Kâº, calcium ionophore II for Ca²âº, sodium ionophore X for Naâº) [72].
- Dissolve in tetrahydrofuran (100 mg/mL) [72].
- Store ISM cocktails at 6°C until use [72].
Measurement and Data Analysis in Complex Biofluids
Protocol: Sensor characterization in biological samples
Equipment: Semiconductor analyzer (e.g., PDA-FS380) or source measurement units for transfer characteristics [58] [72].
Method:
- Prepare calibration standards in artificial biofluid matching target matrix (artificial sweat, saliva, or serum) [72] [67].
- Condition sensors in blank biofluid for 15-30 minutes before measurements.
- Acquire transfer characteristics at fixed drain voltage (-0.4 to -0.6 V) while sweeping gate voltage from -0.6 to 1.2 V [58] [72].
- Measure current-time curves with total testing time of 300 seconds per concentration [72].
- For multi-analyte detection, employ machine learning algorithms (e.g., principal component analysis, support vector machines) to mitigate cross-interference [72].
Protocol: Validation against reference methods
- Collect patient samples (blood, saliva, urine) following institutional ethical guidelines.
- Split samples for parallel analysis using OECT sensors and reference methods (ion chromatography, ICP-MS, ELISA) [73].
- Perform correlation analysis and Bland-Altman plots to assess agreement between methods.
- Account for matrix effects by standard addition or dilution studies in complex biofluids.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential materials for OECT-based clinical sensor development
| Material Category | Specific Examples | Function | Application Notes |
|---|---|---|---|
| Channel Materials | PEDOT:PSS (Clevios PH 1000, SV4) | Mixed ionic-electronic conduction | Add ethylene glycol and cross-linkers for enhanced stability [58] [72] |
| Substrates | Polyethylene naphthalate (PEN), Polyimide (Kapton), Nitrocellulose | Flexible support | PEN offers optical transparency; nitrocellulose for paper-based sensors [58] [72] |
| Electrode Materials | Gold, Silver, Gold-plated copper | Electronic conduction | Gold provides electrochemical stability; silver for printable electrodes [58] [4] |
| Ion-Selective Components | Valinomycin (Kâº), Calcium ionophore II (Ca²âº), Sodium ionophore X (Naâº) | Target recognition | Dissolve in PVC matrix with plasticizer [72] [73] |
| Enzymatic Elements | Glucose oxidase, Lactate oxidase, Alcohol dehydrogenase | Biocatalytic recognition | Stabilize with BSA and cross-link with glutaraldehyde [5] [67] |
| Solid-State Electrolytes | PSSNa, PEA-based gels | Ion conduction in solid state | Enable all-solid-state devices; prevent electrode degradation [73] [4] |
OECT technology has demonstrated significant potential for clinical applications involving complex biofluids and patient samples. The high sensitivity, selectivity, and stability achieved in various biological matrices position OECTs as promising platforms for next-generation diagnostic and monitoring systems. Successful clinical validation requires careful attention to matrix effects, appropriate functionalization strategies, and implementation of advanced data processing techniques to ensure accurate and reliable performance. As fabrication methods continue to advance and standardization protocols emerge, OECT-based biosensors are poised to make substantial contributions to personalized medicine, point-of-care diagnostics, and continuous health monitoring.
Within the field of organic electrochemical transistor (OECT) biosensing research, the functionalization of transducer surfaces represents a critical step that governs the ultimate selectivity, sensitivity, and reliability of the biosensor. A persistent challenge in this domain is the creation of a stable, robust interface for immobilizing biorecognition elements. While self-assembled monolayers (SAMs) derived from thiolated compounds on gold have been the longstanding benchmark for such functionalization, their susceptibility to oxidative degradation compromises the long-term performance of biosensors. The emergence of N-heterocyclic carbene (NHC)-based SAMs presents a promising alternative, offering a potentially superior anchor for bioreceptors on biosensor surfaces. This Application Note provides a comparative benchmark of NHC versus traditional thiol-based SAMs, detailing experimental protocols and presenting quantitative data to guide researchers and scientists in the development of next-generation OECT biosensors.
Quantitative Benchmarking: NHCs vs. Thiols
The performance and properties of NHC and thiol SAMs have been directly compared in several key studies. The data below summarize the critical differentiating factors.
Table 1: Key Performance Indicators for SAMs in Biosensing Applications
| Performance Indicator | N-Heterocyclic Carbenes (NHCs) | Traditional Thiol SAMs | Reference(s) |
|---|---|---|---|
| Bond Dissociation Energy (Au) | ~67 kcal/mol [31] | ~45 kcal/mol [31] | [31] |
| Bond Length (Au) | 2.0 Ã… [31] | 2.2 - 2.6 Ã… [31] | [31] |
| Long-Term Functional Stability | Functional after 24 months of storage at room temperature [31] [35] | Rapid oxidation and degradation under ambient conditions [31] | [31] [35] |
| Stability under Voltammetric Interrogation | Stable within a specific voltage range; desorption outside this range [74] | Broader voltage tolerance before desorption [74] | [74] |
| Thermal Stability | Stable up to ≥573 K [34] | Lower thermal stability [34] | [34] |
| Stability to Chemical Oxidants | Survives exposure to H2O2 [34] | Susceptible to oxidation [31] | [31] [34] |
| Stability in Aqueous Environments | High hydrolytic stability; withstands pH ranges from 2-12 [31] [34] | Susceptible to changes in pH [31] | [31] [34] |
Table 2: Biosensing Performance in a Model OECT System (Biotin-Streptavidin Binding)
| Biosensing Parameter | NHC-Functionalized OECT | Note on Thiol-Based Equivalents | Reference(s) |
|---|---|---|---|
| Target Analytic | Streptavidin (SA) | N/A | [31] [35] |
| Signal Readout | Threshold Voltage Shift (ΔVT) | N/A | [31] [35] |
| Response to Target (SA) | 193 ± 64 mV | N/A | [31] [35] |
| Response to Non-target (BSA) | 62 ± 41 mV | N/A | [31] [35] |
| Selectivity Ratio (SA:BSA) | ~3:1 | N/A | [31] [35] |
| Performance after 24-month storage | ΔVT = 161 ± 30 mV for SA | Thiol SAMs are known to degrade over this timeframe [31] | [31] [35] |
Experimental Protocols
Protocol 1: Fabrication of Aerosol Jet-Printed OECTs
This protocol outlines the fabrication of the core OECT device, which serves as the transducer platform for functionalized SAMs [31].
- Substrate Preparation: Begin with a flexible polyimide substrate. Clean the substrate sequentially in acetone, isopropanol, and deionized water under ultrasonication for 10 minutes each, then dry with a stream of nitrogen.
- Electrode Printing: Load gold nanoparticle (Au NP) ink into the ultrasonic atomizer (UA) of an Optomec Aerosol Jet 5X 3D printer. Print the source, drain, and gate electrodes onto the substrate.
- Electrode Annealing: Anneal the printed Au structures on a hotplate at 280°C for 1 hour to sinter the nanoparticles and achieve high conductivity.
- Channel Deposition: Prepare the PEDOT:PSS channel ink by mixing 94% Heraeus Clevios PH 1000, 5% ethylene glycol (EG), 0.1% dodecylbenzenesulfonic acid (DBSA), and 1% (3-glycidyloxypropyl) trimethoxysilane (GOPS). Deposit the channel material between the source and drain electrodes using the ultrasonic atomizer.
- Channel Annealing: Cure the PEDOT:PSS channel in an oven at 130°C for 20 minutes.
- Insulation Layer Deposition: Dilute UV-curable polydimethylsiloxane (PDMS) with hexanes in a 3:1 volume ratio. Deposit the diluted PDMS over the metal interconnects using the pneumatic atomizer (PA) to prevent electrical shorting with the electrolyte.
- Curing: Cure the PDMS layer by exposing it to UV light during printing, followed by a final thermal anneal at 130°C for 30 minutes.
Protocol 2: NHC SAM Formation and Gate Functionalization
This protocol details the synthesis of the NHC ligand and its application for functionalizing the Au gate electrode of the OECT [31].
- NHC Ligand Synthesis (under inert atmosphere):
- Use Schlenk techniques under a nitrogen environment.
- Dissolve 500 mg of imidazole in 50 mL of anhydrous acetonitrile.
- Add 2.42 mL of 3-bromo-1-(trimethylsilyl)-1-propyne to the imidazole solution.
- The resulting mixture is stirred for a defined period (as per the referenced synthesis) to yield the NHC precursor [31].
- NHC SAM Formation on Au Gate:
- Prepare a solution of the synthesized NHC ligand in a suitable anhydrous solvent (e.g., THF or acetonitrile).
- Immerse the cleaned, aerosol jet-printed Au gate electrode into the NHC solution.
- Allow the SAM to form over several hours at room temperature.
- Remove the electrode from the solution and rinse thoroughly with the solvent and ethanol to remove physisorbed molecules.
- Biofunctionalization via Click Chemistry:
- The NHC monolayer can be further functionalized with biorecognition elements. For instance, a biotin moiety can be attached to the NHC-SAM using copper-catalyzed azide-alkyne cycloaddition (CuAAC) "click" chemistry [31] [34].
- This creates a surface ready to selectively capture the target analyte, such as streptavidin.
Protocol 3: Thiol SAM Formation for Comparative Studies
This standard protocol is provided for generating comparative data against NHC-SAMs [31] [75].
- Substrate Cleaning: Clean gold electrodes via oxygen plasma treatment or by piranha solution (Caution: piranha is highly corrosive and must be handled with extreme care), followed by rinsing with copious amounts of water and ethanol.
- SAM Formation: Prepare a 1 mM solution of the desired thiolated molecule (e.g., a biotin-terminated alkanethiol) in high-purity ethanol.
- Immerse the clean Au substrates in the thiol solution. SAM formation can be performed at room temperature for a minimum of 12-18 hours.
- Rinsing and Drying: Remove the substrates from the solution, rinse thoroughly with ethanol to remove unbound thiols, and dry under a stream of inert gas (e.g., nitrogen or argon).
Workflow and Functionalization Strategies
The following diagrams illustrate the core experimental workflow and the strategic placement of the functionalized SAM within an OECT biosensor.
The primary biosensing mechanism for the described OECT platform involves functionalizing the gate electrode. The binding of a target analyte to the bioreceptors on the gate alters the interfacial potential, which is efficiently amplified by the OECT and read out as a pronounced shift in the transistor's threshold voltage (ΔVT) [31] [5].
Table 3: The Scientist's Toolkit: Essential Research Reagent Solutions
| Reagent / Material | Function in Experiment | Specification / Notes |
|---|---|---|
| Gold Nanoparticle (Au NP) Ink | Forms the conductive gate, source, and drain electrodes via aerosol jet printing. | UT Dots, Inc. ink; requires annealing at 280°C for 1 hour [31]. |
| PEDOT:PSS (Clevios PH 1000) | The active semiconducting polymer channel of the OECT. | Mixed with EG, DBSA, and GOPS for printability and stability [31]. |
| NHC Ligand Precursor | Forms the robust self-assembled monolayer on the Au gate surface. | Synthesized from imidazole and 3-bromo-1-(trimethylsilyl)-1-propyne [31]. |
| Biotin-based Azide | The "bait" molecule for demonstrating selective capture of streptavidin. | Attached to the alkyne-functionalized NHC SAM via CuAAC click chemistry [31] [34]. |
| Polydimethylsiloxane (PDMS) | Dielectric insulation layer to protect metal interconnects from the electrolyte. | UV-curable type; diluted with hexanes for optimal deposition [31]. |
The quantitative data and detailed protocols presented in this application note robustly demonstrate that NHC-based SAMs offer a formidable advantage over traditional thiol-based SAMs for OECT biosensing applications, primarily due to their exceptional long-term stability. The strong covalent Au-C bond in NHCs translates to biosensors that retain high performance over extended periods, as evidenced by the minimal signal degradation after 24 months of storage. While thiol chemistry remains a versatile and well-understood tool, its susceptibility to oxidation is a significant limitation for the development of reliable, commercial-grade biosensors. The adoption of NHC functionalization, particularly in conjunction with the high signal amplification of OECTs, paves the way for a new class of durable and reliable biosensors for demanding applications in point-of-care diagnostics, continuous health monitoring, and pharmaceutical development.
Conclusion
Organic Electrochemical Transistors represent a paradigm shift in biosensing, merging high sensitivity and low operating voltage with unprecedented compatibility with flexible and wearable form factors. The synthesis of knowledge across the four intents confirms that advancements in scalable fabrication, robust functionalization chemistries like NHCs, and innovative concepts for reusability are rapidly addressing the traditional limitations of stability and cost. The future of OECTs lies in their integration into multifunctional, closed-loop diagnostic and therapeutic systems. Key directions include the development of novel OMIECs for specific biomarkers, the seamless incorporation of AI for data analysis in point-of-care settings, and the transition toward large-scale clinical trials to validate their efficacy in personalized medicine and continuous health monitoring. The trajectory of OECT technology promises to fundamentally reshape biomedical research and clinical diagnostics.
References
- 1. A guide for the characterization of organic electrochemical ... [https://pubs.rsc.org/en/content/articlehtml/2023/cs/d2cs00920j]
- 2. Organic electrochemical transistor [https://en.wikipedia.org/wiki/Organic_electrochemical_transistor]
- 3. Biological Applications of Organic Electrochemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6514146/]
- 4. A fully-integrated flexible in-sensor computing circuit based ... [https://www.nature.com/articles/s41528-025-00472-x]
- 5. Biomolecule sensors based on organic electrochemical ... [https://www.nature.com/articles/s41528-025-00383-x]
- 6. Organic Electrochemical Transistors for Biomarker Detections [https://pmc.ncbi.nlm.nih.gov/articles/PMC11251571/]
- 7. Organic electrochemical transistors as novel biosensing ... [https://pubs.rsc.org/en/content/articlelanding/2022/tb/d1tb01584b]
- 8. Transient Response and Ionic Dynamics in Organic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11219702/]
- 9. New method developed to dramatically enhance bioelectronic ... [https://news.rice.edu/news/2025/new-method-developed-dramatically-enhance-bioelectronic-sensors]
- 10. Stretchable all-gel organic electrochemical transistors [https://www.nature.com/articles/s41467-025-59240-0]
- 11. Glucose selective textile OECT based on molecularly ... [https://www.sciencedirect.com/science/article/pii/S0925400525004150]
- 12. Fiber-based organic electrochemical transistors for re- ... [https://www.nature.com/articles/s41528-025-00488-3]
- 13. Functional Organic Electrochemical Transistor-Based ... [https://www.mdpi.com/2227-9040/12/11/236]
- 14. Recent Progress in Organic Electrochemical Transistor ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11274720/]
- 15. Fibre-based organic electrochemical transistors: principle, ... [https://www.nature.com/articles/s41528-025-00417-4]
- 16. Solid-state organic electrochemical transistors (OECTs) based ... [https://pubs.rsc.org/en/content/articlehtml/2025/ta/d4ta05288a]
- 17. Biological Sensing Using Vertical MoS2-Graphene ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12190709/]
- 18. Melanin/PEDOT:PSS blend as organic mixed ionic electronic ... [https://pubs.rsc.org/en/content/articlehtml/2023/ma/d3ma00573a]
- 19. Flexible organic electrochemical transistors for bioelectronics [https://www.sciencedirect.com/science/article/pii/S2666386423005027]
- 20. PEDOT:PSS versus Polyaniline: A Comparative Study of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10747145/]
- 21. Robust and flexible organic electrochemical transistors ... [https://www.nature.com/articles/s41528-025-00457-w]
- 22. Backbone Tailoring Enables High-Performance and Stable ... [https://pubmed.ncbi.nlm.nih.gov/41045119/]
- 23. Electrical characteristics of porous-structured PEDOT:PSS ... [https://link.springer.com/article/10.1557/s43579-025-00872-0]
- 24. Integrated Perspective on Functional Organic ... [https://www.mdpi.com/2227-9040/13/6/215]
- 25. Flexible Pcb in the Real World: 5 Uses You'll Actually ... [https://www.linkedin.com/pulse/flexible-pcb-real-world-5-uses-youll-actually-r82bc/]
- 26. Organic Electrochemical Transistors Monolithically ... [https://pubmed.ncbi.nlm.nih.gov/40836871/]
- 27. Essential DFM Strategies for Flex PCB Designers in 2025 [https://www.bestfpc.com/news/essential-dfm-strategies-for-flex-pcb-designers-in-2025.html]
- 28. Flex PCB Design Guidelines (2025): Expert Guide to ... [https://www.richpcba.com/blogs/flex-pcb-design-guidelines/]
- 29. Flex PCB Design Guidelines: Optimizing Layout for ... [https://www.allpcb.com/allelectrohub/flex-pcb-design-guidelines-optimizing-layout-for-manufacturing]
- 30. Functional gate modification for OECT-based sensors [https://www.sciencedirect.com/science/article/abs/pii/S2468519425004239]
- 31. Long-term stability of N-heterocyclic carbene (NHC ... [https://www.frontiersin.org/journals/sensors/articles/10.3389/fsens.2024.1430958/full]
- 32. Long-term stability of N-heterocyclic carbene (NHC ... [https://www.semanticscholar.org/paper/Long-term-stability-of-N-heterocyclic-carbene-%28NHC%29-Fan-Kang/3795df4ac4d0d97b5fbb3cfb3cb6feb547d1181e]
- 33. N-heterocyclic carbenes as clickable molecular anchors for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12495314/]
- 34. N-heterocyclic carbenes as clickable molecular anchors for ... [https://pubs.rsc.org/en/content/articlehtml/2025/sc/d5sc03908h]
- 35. Long-term stability of N-heterocyclic carbene (NHC ... [https://doaj.org/article/e8859692771641d0918ebb9cbe0a97c8]
- 36. High-Precision Field- Effect Transistor Biosensor for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11852787/]
- 37. Solid-state organic electrochemical transistors (OECTs ... [https://pubs.rsc.org/en/content/articlelanding/2025/ta/d4ta05288a]
- 38. blended EGylated conjugated and radical polymers in ... [https://www.nature.com/articles/s41528-025-00412-9]
- 39. Expanding the potential of biosensors: a review on organic ... [https://www.materialsfutures.org/article/doi/10.1088/2752-5724/ace3dd]
- 40. Conjugated polyelectrolyte-aptamer hybrid for organic- ... [https://www.sciencedirect.com/science/article/pii/S2666386425005648]
- 41. Sensitive organic electrochemical transistor biosensors [https://www.sciencedirect.com/science/article/abs/pii/S095656632200731X]
- 42. Benchmarking organic electrochemical transistors for plant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9355396/]
- 43. Smart sweat glucose detection system based on organic ... [https://www.sciencedirect.com/science/article/pii/S187220402400001X]
- 44. From Enzymatic Dopamine Biosensors to OECT Biosensors of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10452593/]
- 45. Wearable Sensor System for Detection of Lactate in Sweat [https://www.nature.com/articles/s41598-018-33565-x]
- 46. Nanostructured Materials in Glucose Biosensing [https://pmc.ncbi.nlm.nih.gov/articles/PMC12563017/]
- 47. Polymer-Based Electrochemical Sensors for the Diagnosis of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12106273/]
- 48. Biosensors, Volume 15, Issue 5 (May 2025) – 69 articles [https://www.mdpi.com/2079-6374/15/5]
- 49. New method developed to dramatically enhance ... [https://www.sciencedaily.com/releases/2025/02/250226125012.htm]
- 50. New progress in achieving renewable high sensitivity ... [https://www.senther.com/en/News/233.html]
- 51. Reusable OECT biosensor - Research Communities [https://communities.springernature.com/posts/drug-make-oect-biosensor-reusable]
- 52. A drug-mediated organic electrochemical transistor for ... [https://pubmed.ncbi.nlm.nih.gov/39112738/]
- 53. The Rising of Flexible Organic Electrochemical Transistors ... [https://pubmed.ncbi.nlm.nih.gov/39829276/]
- 54. Experimental design of stencil-printed high-performance ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10711736/]
- 55. Flexible circuits engineered for complex and extreme ... [https://www.oaepublish.com/articles/ss.2025.69]
- 56. A focused review on organic electrochemical transistors [https://www.sciencedirect.com/science/article/abs/pii/S0026265X24018496]
- 57. Rapid prototyping of 3D Organic Electrochemical ... [https://www.nature.com/articles/s41598-020-70365-8]
- 58. A Printable OECT for Simple Integration in Nitrocellulose ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12560127/]
- 59. Organic Electronics in Biosensing : A Promising Frontier for Medical... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10669243/]
- 60. Frontiers | Biological Applications of Organic Electrochemical... [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2019.00313/full]
- 61. Biosensor development using organic... | ERA [https://era.library.ualberta.ca/items/7764d423-7707-40d5-aa78-0dcc0b06900f]
- 62. the critical role of volumetric capacitance in predictive 2D ... [https://www.nature.com/articles/s41528-025-00482-9]
- 63. A high-performance organic electrochemical transistor ... [https://pubs.rsc.org/en/content/articlelanding/2023/tc/d3tc01491f]
- 64. Long-Term Stability in Electronic Properties of Textile ... [https://www.mdpi.com/1996-1944/16/5/1861]
- 65. Balancing performance and stability characteristics in ... [https://pubmed.ncbi.nlm.nih.gov/40245610/]
- 66. Reconfiguration of organic electrochemical transistors for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11294360/]
- 67. Critical perspectives on electrochemical biosensing strategies ... [https://pubs.rsc.org/en/content/articlehtml/2025/ay/d5ay00941c]
- 68. Revealing the Impact of Gel Electrolytes on ... [https://www.mdpi.com/2310-2861/11/3/202]
- 69. OFET and OECT, Two Types of Organic Thin-Film ... [https://ieee-sensorsalert.org/articles/ofet-and-oect-two-types-of-organic-thin-film-transistor-used-in-glucose-and-dna-biosensors-a-review/]
- 70. Stretchable Transistors Enable In-Sensor Edge Computing in ... [https://www.embedded.com/stretchable-transistors-enable-in-sensor-edge-computing-in-wearable-technology/]
- 71. Sterilizable vertical n-type organic electrochemical ... [https://www.sciencedirect.com/science/article/abs/pii/S0927796X25000804]
- 72. A Flexible Multi-Ion Detection System Based on Organic ... [https://www.mdpi.com/2079-9292/14/5/1023]
- 73. Implantable Ionâ€Selective Organic Electrochemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12591102/]
- 74. The Robust Stability of N-Heterocyclic Carbene Monolayers [https://advanceseng.com/advancing-electrochemical-sensing-robust-stability-heterocyclic-carbene-monolayers/]
- 75. [PDF] An electrochemical comparison of thiolated self ... [https://www.semanticscholar.org/paper/An-electrochemical-comparison-of-thiolated-(SAM)-in-Piper-Corrigan/7ff3701eddf2d262714d7a9301638ff64ae19bfb]